
Personalized T-Cell therapies in the treatment of hematological malignancies
Introduction
Cancer is still one of the most difficult diseases to cure, and conventional treatments such as chemotherapy, radiation, and surgery have yielded limited success and severe side effects. Immunotherapy has changed the face of cancer treatment by using the immune system of the body to destroy cancer cells. Among these treatments, Chimeric Antigen Receptor (CAR) T-cell therapy has become a revolutionary technique, especially for hematologic malignancies. It gives a complete analysis of CAR-T cell therapy, its working, clinical achievements, hurdles, and future perspective.
Hematological malignancies
A considerable percentage of hematological cancers have restricted therapy alternatives. Combinatorial therapies, including chemotherapy combined with targeted therapy via small molecules or monoclonal antibodies and/or hematopoietic stem cell transplantation (HSCT), have resulted in sustained remission or potential cure in certain hematological malignancies. Although HSCT is presently regarded as the primary treatment for the majority of hematological malignancies, it may be associated with significant problems.
Notably, the graft-versus-leukemia response (GVL) in hematopoietic stem cell transplantation (HSCT) has been documented to enhance anticancer efficacy. This observation offers substantial evidence that donor immune cells can markedly eradicate malignant host cells in leukemia, lymphoma, and multiple myeloma. Consequently, regulating the immune system may serve as a viable treatment strategy to address hematological malignancies.
Cytotoxic T lymphocytes (CTLs) are a crucial subset of effector T-cells that facilitate antitumor immunity by causing cytolysis or death of malignant cells in an HLA-dependent way. Hematological malignant cells can exploit several mechanisms to circumvent CTL-mediated immunity and develop resistance to existing combinational treatments, leading to recurrence or treatment failure.
The immune evasion of hematological malignancies may involve compromised tumor antigen processing and presentation by tumor cells, malfunction of antigen presenting cells (APCs), and poor costimulation and/or coinhibitory T-cell mediated pathways associated with immune checkpoint inhibition. Furthermore, the proliferation of suppressive immune cells, tumor-altered metabolism, the secretion of regulatory soluble molecules within the tumor microenvironment, and the downregulation of tumor cell surface antigens also promote immune evasion from the CTL-mediated response.
Addressing tumor immune evasion may be pivotal in the effective treatment of certain hematological malignancies. Consequently, comprehending the intricate pathways of immune evasion is essential for the advancement of innovative immunotherapy strategies for these cancers.
In solid tumors like melanoma, tumor-infiltrating lymphocytes extracted from tumor tissues, when expanded ex vivo and subsequently reinfused into the patient, elicited a partial anticancer response. Although allogeneic HSCT is similarly effective in treating or curing most hematological malignancies, both allogeneic HSCT and the adoptive transfer of tumor-infiltrating lymphocytes may result in lethal consequences or treatment failure.
This predicament has compelled cancer immunologists to explore supplementary methods to modify CTLs for the specific recognition and destruction of tumor cells by mitigating tumor immune evasion. At present, genetically modified T-cell-based adoptive immunotherapies, particularly engineered chimeric antigen receptor (CAR) gene-transduced T-cells (CAR-T) and T-cell receptor (TCR) gene-transduced T-cells (TCR-T), represent significant developments in clinical cancer treatment.
CAR is a chimeric protein consisting of an external single-chain variable fragment (scFv) generated from an antibody for antigen recognition, coupled with an intracellular T-cell activation domain. CAR-expressing T-cells can attach to particular antigens and eliminate tumor cells in an HLA-independent fashion. Numerous clinical trials have shown that CAR-T cell-based adoptive immunotherapy yields a prolonged remission in hematological malignancies that surpasses existing standard combination treatments.
In theory, CAR recognition is confined to surface antigens inside the framework of HLA molecules. Conversely, modified TCR gene-transduced T-cells can identify intracellular proteins that are processed and presented by antigen presentation cells (APCs) or tumor cells in an HLA-dependent fashion. Multiple lines of evidence indicate that hematological malignancies develop tumor-associated mutations, some of which may produce neoantigens that can affect the antitumor response and act as innovative targets for adoptive immunotherapy.
Neoantigen-specific CTLs are believed to eliminate tumor cells through the HLA-dependent presentation of peptides produced from neoantigens. Regrettably, neoantigen-specific cytotoxic T lymphocytes cannot be activated within the tumor-altered milieu. Engineered T-cells expressing neoantigen-specific TCRs can be expanded ex vivo and transfused into the patient, thereby establishing a targeted TCR-T-cell-mediated immunity to eradicate malignant cells. Consequently, the recent progress in genetically engineered T-cell immunotherapy represents a more targeted method for the treatment or eradication of hematological malignancies.
CAR-T and TCR-T cell-based immunotherapies, which can disrupt some pathways associated with immune evasion, may possess limitations regarding their adverse effect profiles. Consequently, the integration of adoptive transfer of CAR-T or TCR-T cells with additional appropriate interventions, such as chemotherapy, immune checkpoint blockade suppression, and/or cytokine treatment, may have a synergistic effect by concurrently disrupting numerous pathways of immune evasion. The swift progress in genome editing and gene transfer technologies may offer a potential foundation for refining CAR-T or TCR-T cell-based immunotherapies to boost immune response through the modification of gene expression.
To summarize current findings regarding genetically modified T-cell-based adoptive immunotherapies for hematological malignancies, we will first outline the existing knowledge of the various mechanisms employed by malignancies for immune evasion to elude recognition by cytotoxic T lymphocytes (CTLs). Subsequently, we will present a comprehensive analysis of the utilization of CAR-T treatment and neoantigen-specific TCR-T cell adoptive immunotherapeutics in addressing immune evasion. Ultimately, we will assess strategies aimed at alternative mechanisms of immune evasion to enhance CAR-T or TCR-T cell-based immunotherapy.
Understanding CAR T Cell therapy
CAR-T cell therapy is a form of adoptive cell transfer immunotherapy that involves modifying a patient’s T-cells to enhance their ability to recognize and attack cancer cells. The process involves several key steps:
- T-cell Collection: The patient’s blood is drawn, and T-cells (a type of white blood cell crucial for immune responses) are extracted through a process called leukapheresis.
- Genetic Modification: The T-cells are genetically engineered to express a synthetic receptor known as a Chimeric Antigen Receptor (CAR), which enables them to specifically recognize cancer cells.
- Cell Expansion: The modified T-cells are expanded in a laboratory to create millions of potent cancer-fighting cells.
- Infusion into the Patient: The engineered T-cells are infused back into the patient’s bloodstream, where they actively seek and destroy cancer cells.
- Long-Term Immune Response: These modified T-cells can persist in the body, providing long-term protection against cancer recurrence.
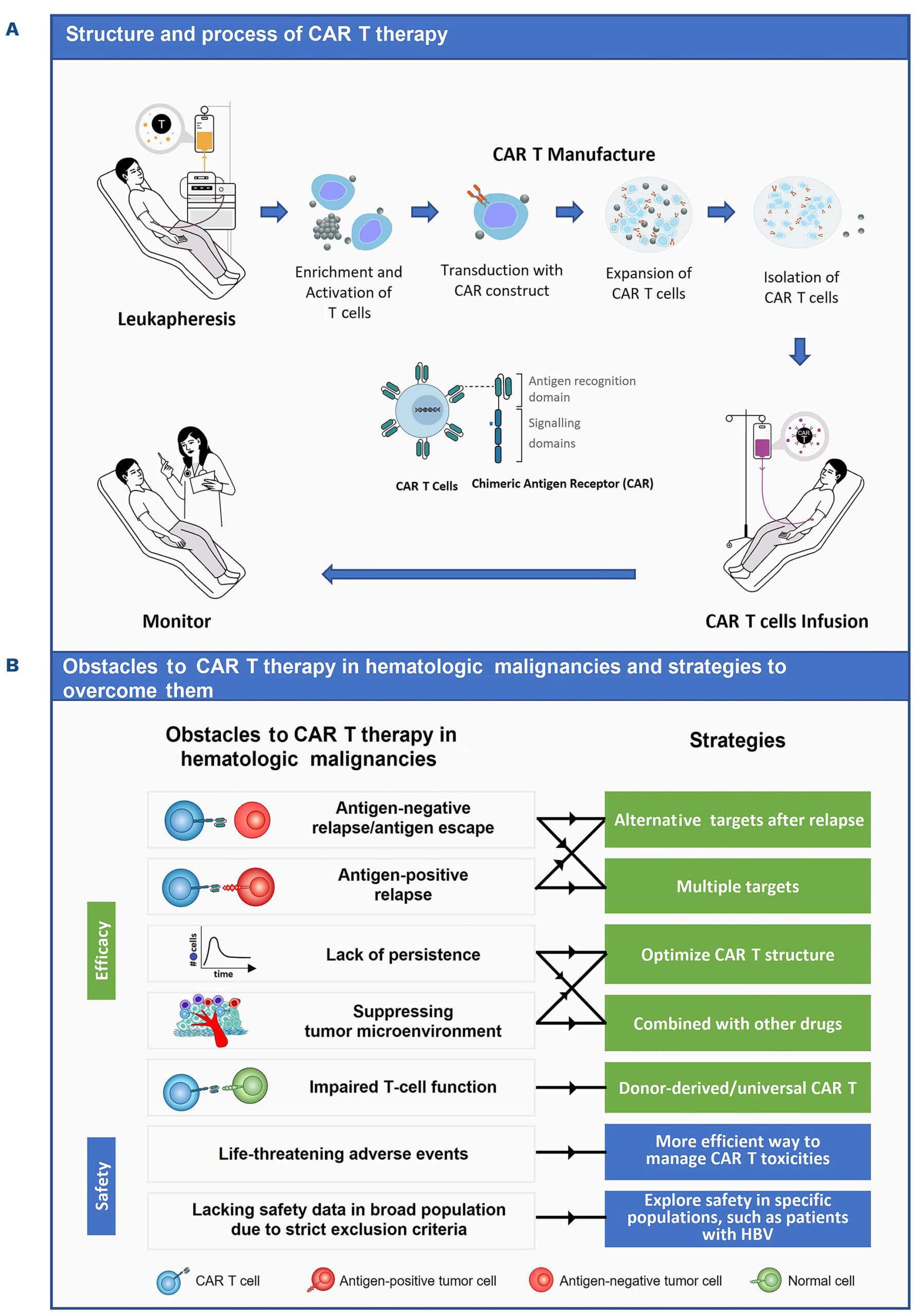
Pic: CAR T Cell therapy process
Targeting CD19: A key milestone
The majority of successful CAR-T therapies target CD19, a protein found on the surface of B-cells, including cancerous B-cells in diseases such as:
- Acute Lymphoblastic Leukemia (ALL)
- Chronic Lymphocytic Leukemia (CLL)
- Non-Hodgkin’s Lymphoma (NHL)
Clinical trials have demonstrated remarkable success rates, with many patients achieving complete remission even after failing conventional treatments.
FDA approved CAR T Cell therapies
The FDA has approved several CAR-T cell therapies for different types of blood cancers:
- Tisagenlecleucel (Kymriah): Approved for pediatric and young adult patients with B-cell precursor acute lymphoblastic leukemia (ALL).
- Axicabtagene ciloleucel (Yescarta): Approved for adults with relapsed or refractory large B-cell lymphoma.
- Brexucabtagene autoleucel (Tecartus): Approved for mantle cell lymphoma (MCL).
- Lisocabtagene maraleucel (Breyanzi): Approved for relapsed/refractory large B-cell lymphoma.
These approvals underscore the effectiveness of CAR-T therapy, particularly for patients who have exhausted other treatment options.
Challenges and limitations of CAR T Cell therapy
Despite its success, CAR-T therapy comes with significant challenges:
1. Cytokine Release Syndrome (CRS)
CRS is a potentially life-threatening inflammatory response that occurs when CAR-T cells become overly activated and release excessive cytokines. Symptoms include:
- High fever
- Low blood pressure
- Organ dysfunction
CRS is typically managed with corticosteroids and the IL-6 inhibitor Tocilizumab, which helps reduce inflammation.
2. Neurotoxicity
Some patients experience neurological side effects, such as confusion, seizures, or speech difficulties. The exact cause remains unclear, but careful monitoring and supportive care can mitigate risks.
3. High Costs and Manufacturing Complexity
CAR-T therapy is expensive, with costs exceeding $400,000 per treatment. The complex manufacturing process, which requires personalized cell engineering and rigorous quality control, adds to the expense and limits accessibility.
4. Challenges in Solid Tumors
While CAR-T therapy has been highly effective against blood cancers, its application in solid tumors remains a challenge due to:
- Lack of unique tumor-specific antigens
- Immunosuppressive tumor microenvironment
- Limited T-cell infiltration into tumors
Innovations and future direction
Researchers are exploring several strategies to overcome these limitations:
1. Next-Generation CAR-T Cells
Scientists are developing dual-targeting CAR-T cells that can recognize multiple cancer antigens simultaneously, reducing the risk of tumor escape.
2. Allogeneic (“Off-the-Shelf”) CAR-T Therapy
Unlike traditional CAR-T therapy, which uses a patient’s own cells, allogeneic CAR-T therapy utilizes donor-derived T-cells. This approach could reduce costs and expand availability.
3. Combination Therapies
CAR-T therapy is being combined with immune checkpoint inhibitors (e.g., PD-1 inhibitors) to enhance T-cell persistence and improve outcomes.
4. Engineering CAR-T Cells to Overcome the Tumor Microenvironment
New strategies include:
- Modifying CAR-T cells to secrete cytokines that counteract immune suppression within tumors.
- Designing CAR-T cells with enhanced metabolic fitness for better persistence.
Conclusion
A full understanding of the many mechanisms of tumor immune evasion is essential for the development of genetically engineered T-cell-based adoptive immunotherapeutics to effectively treat or cure patients with hematologic cancers. Clinical trials of CAR-T and TCR-T cell therapies in hematological malignancies and solid tumors, including melanoma, present numerous successful cases that substantiate the efficacy and safety of this therapeutic approach in clinical settings.
Future strategies must meticulously address three key aspects:
(1) the identification of novel targets, encompassing tumor-specific surface molecules and neoantigens, alongside the integration of omics science with immunology;
(2) a comprehensive understanding of the collaboration and interaction between T-cell-based adoptive immunotherapies and other treatments to formulate an optimal combinatorial therapy; and
(3) the implementation of effective measures utilizing genome editing and gene transfer technologies to improve efficacy and minimize toxicity, thereby promoting the advancement and clinical application of this swiftly evolving technology.

Dr. Creed M Stary
Dr. Creed M. Stary is an Associate Professor of Anesthesiology, Perioperative and Pain Medicine at Stanford University School of Medicine. He also holds a courtesy appointment as an Associate Professor of Ophthalmology.
Dr. Stary's educational background includes:
PhD in Biomedicine from UC San Diego (2006)
MD from University of California San Diego School of Medicine (2008)
Residency in Anesthesiology at UCSD (2012)
Board Certification from the American Board of Anesthesiology (2014)
His research interests focus on finding new strategies to promote neuronal survival and improve functional outcomes following brain injury. Specifically, he studies the role of non-coding RNAs in:
Astrocyte-mediated protection and recovery of brain function after ischemic injury
Gender differences in severity of injury and recovery from stroke
Dr. Stary is involved in several professional organizations, including:
Association of University Anesthesiologists
American Heart Association
International Anesthesia Research Society
Society for Neuroscience
American Society of Anesthesiologists
He has received multiple research grants and awards, including NIH R01 grants for his work on cofilin signaling in hemorrhagic stroke and non-coding RNA regulation of sex differences in stroke.
At Stanford, Dr. Stary serves as the Assistant Clinical Director for Anesthesiology at the Byers Eye Institute.
- This author does not have any more posts.
Related Posts
- Comments Closed
- February 17th, 2025


- Datopotamab deruxtecan-dlnk is approved by the USFDA for EGFR-mutated non-small cell lung cancer
- Tafasitamab-cxix is approved by the USFDA for relapsed or refractory follicular lymphoma
- PiggyBac Transposon System: A Revolutionary Tool in Cancer Gene Therapy
- Breakthrough Treatments for Advanced Breast Cancer in 2025
- Neoadjuvant and adjuvant pembrolizumab is approved by the USFDA for resectable locally advanced head and neck squamous cell carcinoma
- Mitomycin intravesical solution is approved by the USFDA for recurrent low-grade intermediate-risk non-muscle invasive bladder cancer
- Taletrectinib is approved by the USFDA for ROS1-positive non-small cell lung cancer
- Darolutamide is approved by the USFDA for metastatic castration-sensitive prostate cancer
- Atezolizumab Plus Chemotherapy Improves Survival in Advanced-Stage Small-Cell Lung Cancer: Insights from the IMpower133 Study
- Satri-cel CAR T-Cell Therapy: A New Era in Gastric Cancer Treatment
- AI & Technology (12)
- Aids cancer (4)
- Anal cancer (9)
- Appendix cancer (3)
- Basal cell carcinoma (1)
- Bile duct cancer (7)
- Biotech Innovations (19)
- Bladder cancer (12)
- Blood cancer (60)
- Bone cancer (12)
- Bone marrow transplant (47)
- Brain Cancer (1)
- Breakthrough Research (17)
- Breast Cancer (53)
- Cancer Guides (10)
- Cancer News and Updates (54)
- Cancer Treatment Abroad (286)
- Cancer treatment in China (316)
- Cancer Treatments (12)
- Cancer Types (5)
- Cancer Warriors (1)
- CAR T Protocols (2)
- CAR T-Cell therapy (135)
- Cervical cancer (40)
- Chemotherapy (55)
- Childhood cancer (2)
- Cholangiocarcinoma (3)
- Clinical trials (15)
- Colon cancer (96)
- Diagnosis & Staging (4)
- Doctors & Researchers (76)
- Drug Approvals (100)
- Drugs (80)
- Endometrial cancer (10)
- Esophageal cancer (15)
- Eye cancer (9)
- For Doctors and Researchers (12)
- Gall bladder cancer (3)
- Gastric cancer (29)
- Gene therapy (5)
- Glioblastoma (7)
- Glioma (10)
- Global Trial News (5)
- Gynecological cancer (2)
- Head and neck cancer (19)
- Hemato-Oncologist (1)
- Hematological Disorders (52)
- Hospital Reviews (3)
- How to Participate (6)
- Immunotherapy (34)
- Kidney cancer (10)
- Laryngeal cancer (1)
- Leukemia (49)
- Liver cancer (101)
- Lung cancer (82)
- Lymphoma (52)
- MDS (2)
- Melanoma (9)
- Merkel cell carcinoma (1)
- Mesothelioma (5)
- Myeloma (25)
- Myths vs Facts (5)
- Neuroblastoma (7)
- NK-Cell therapy (13)
- Nutrition (1)
- Ongoing Trials (11)
- Oral cancer (12)
- Ovarian Cancer (14)
- Pancreatic cancer (43)
- Paraganglioma (6)
- Patient Testimonials (1)
- Penile cancer (1)
- Prostrate cancer (11)
- Proton therapy (28)
- Radiotherapy (56)
- Recovery Tips (2)
- Rectal cancer (58)
- Research Insights (8)
- Sarcoma (14)
- Skin Cancer (13)
- Spine surgery (24)
- Stomach cancer (40)
- Success Stories (1)
- Surgery (102)
- Systemic mastocytosis (1)
- T Cell immunotherapy (7)
- Targeted therapy (9)
- Testicular cancer (5)
- Thoracic surgery (2)
- Throat cancer (6)
- Thyroid Cancer (15)
- Treatment Cost (1)
- Treatment in China (969)
- Treatment in India (1,273)
- Treatment in Israel (652)
- Treatment in Malaysia (425)
- Treatment in Singapore (321)
- Treatment in South Korea (305)
- Treatment in Thailand (291)
- Treatment in Turkey (272)
- Treatment Planning (151)
- Trial Results (2)
- Uncategorized (105)
- Urethral cancer (9)
- Urosurgery (14)
- Uterine cancer (4)
- Vaginal cancer (6)
- Vascular cancer (5)
- Vulvar cancer (1)

Adoptive Cell Transfer, CAR T-Cell Therapy, Hematological Malignancies, hematopoietic stem cell transplantation, Immunotherapy, Neoantigen-Specific T-Cells, Personalized T-Cell Therapies, T-Cell Receptor (TCR) Therapy
CancerFax is the most trusted online platform dedicated to connecting individuals facing advanced-stage cancer with groundbreaking cell therapies.
Send your medical reports and get a free analysis.
🌟 Join us in the fight against cancer! 🌟
Привет,
CancerFax — это самая надежная онлайн-платформа, призванная предоставить людям, столкнувшимся с раком на поздних стадиях, доступ к революционным клеточным методам лечения.
Отправьте свои медицинские заключения и получите бесплатный анализ.
🌟 Присоединяйтесь к нам в борьбе с раком! 🌟