CAR T-Cell Therapy In Malaysia
Planning to visit Malaysia for CAR T treatment?
Get an estimate from the top hospitals in Malaysia.
CAR T cell therapy, a groundbreaking immunotherapy, is making strides in Malaysia’s medical landscape. This innovative treatment involves genetically modifying a patient’s T cells to recognize and attack cancer cells. Although initially available primarily in developed countries, Malaysia’s healthcare sector is embracing this cutting-edge approach. Several hospitals and research centers are exploring CAR T cell therapy’s potential, offering hope for patients with certain types of blood cancers, like leukemia and lymphoma. Challenges persist, including cost and infrastructure, but collaborations between institutions and industry aim to broaden access. As Malaysia advances in biotechnology and healthcare, CAR T cell therapy promises a transformative impact on cancer care.
MALAYSIAN Genomics Resource Centre Bhd (MGRC) hopes to make its chimeric antigen receptor (CAR) T-Cell therapy treatment available in Malaysia for under RM200,000 i.e around $ 45,000 USD, which is a fraction of the cost of therapy in Europe and the United States. Hope the CAR T-Cell therapy in Malaysia will bring cheers to thousands of patients who are hoping to get this breakthrough treatment.
Image: CAR T Cell therapy is been developed for some types of solid tumors too
According to Malaysian Genomics CEO Sasha Nordin, the company has teamed with China’s ICARTAB Biomedical Co Ltd, which is developing the technology exclusively for solid malignancies, despite the fact that similar treatments for liquid or blood diseases are already available in Western countries.
“Improving access to innovative items is part of our mandate.” We were able to reach an agreement that allows us to make it available to patients in Southeast Asia for a fraction of the price.
In an interview with The Malaysian Reserve yesterday, he said, “We are working on making it available for less than RM200,000 versus US$400,000 (RM1.61 million) at minimum in Europe and the US.”
MGRC will be the official distributor of CAR T-Cell treatment in Singapore, Brunei, Indonesia, Thailand, Vietnam, Cambodia, and Laos, in addition to Malaysia.
According to Sasha Omar, the company presently has medicines for six of the region’s top ten malignancies, including liver, pancreatic, mesothelioma, oesophagus, brain, and stomach tumours. He claims that CAR T-cell immunotherapy for solid cancer is not a mass-produced therapeutic.
Every treatment in the therapy is unique to the patient since it includes extracting the patient’s cells and allowing the patient’s T-cell, an important white blood cell in the immune system, to recognise the tumour.
The T-cell will then be reinfused into the patient’s body to detect and fight the malignant cell, preventing it from spreading further.
“Every time we meet a patient, we have to go through a qualification process to evaluate if they are a good candidate for CAR T-Cell therapy.” If they meet all of the criteria, we will produce T-cells exclusively for that patient.
“That is why we believe it is critical to make it considerably more affordable.” We can control the pricing a little better by fitting our own lab,” he said.
Sasha Omar stated that the company’s new laboratory will be operational by the end of March this year, and that activities will resume as soon as possible to accommodate the demand for the therapy.
The new 12,000 sq ft area will be in Kota Damansara, with the laboratory taking up around 7,000 sq ft of the space and the office structure taking up the remaining space.
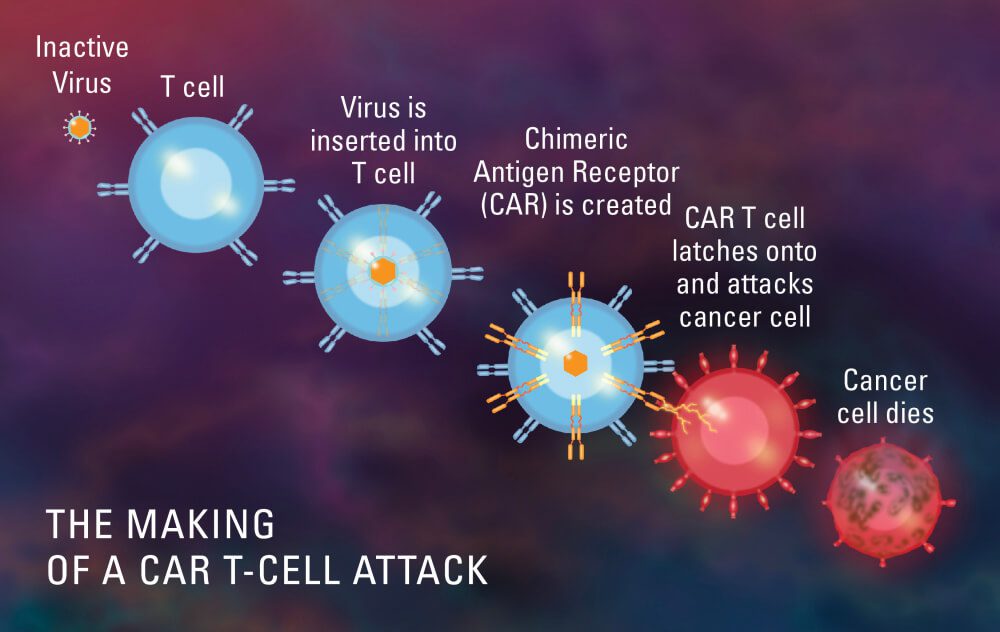
Newly developed drugs, such as CAR-T (chimeric antigen receptor T-cell), are life-changing medications for people who have exhausted their possible treatment options. These cutting-edge treatments are provided in non-traditional ways, including a long, complicated, and highly organized series of activities inside the healthcare provider’s facilities. Providers will benefit from these treatments in a variety of ways, from enhancing their reputation as cutting-edge organization’s to providing high-profit-generating programmes. However, delivering these latest advanced treatments poses significant financial and operating challenges if not carefully assessed and prepared.
What is CAR T-Cell therapy (Chimeric antigen receptors)?
CAR T-Cell therapy is a form of immunotherapy that uses specially modified T-cells which are part of our immune system to fight cancer. A sample of patients T cells are collected from the blood, then it is modified to produce special structures called chimeric antigen receptors (CAR) on their surface. When these modified CAR cells are reinfused in the patient, these new cells attack the specific antigen and kill the tumor cells.
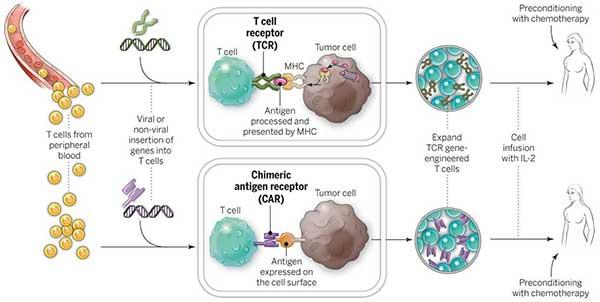
How does CAR T-Cell therapy work?
CAR T-cell therapy takes help from body’s own immune system to attack and kill cancer cells. This is done by removing some specified cells from the blood of the patient, modifying them in the lab and re-injecting them into the patient. CAR T-cell therapy has produced very encouraging results in Non-Hodgkin lymphoma and thus approved by FDA.
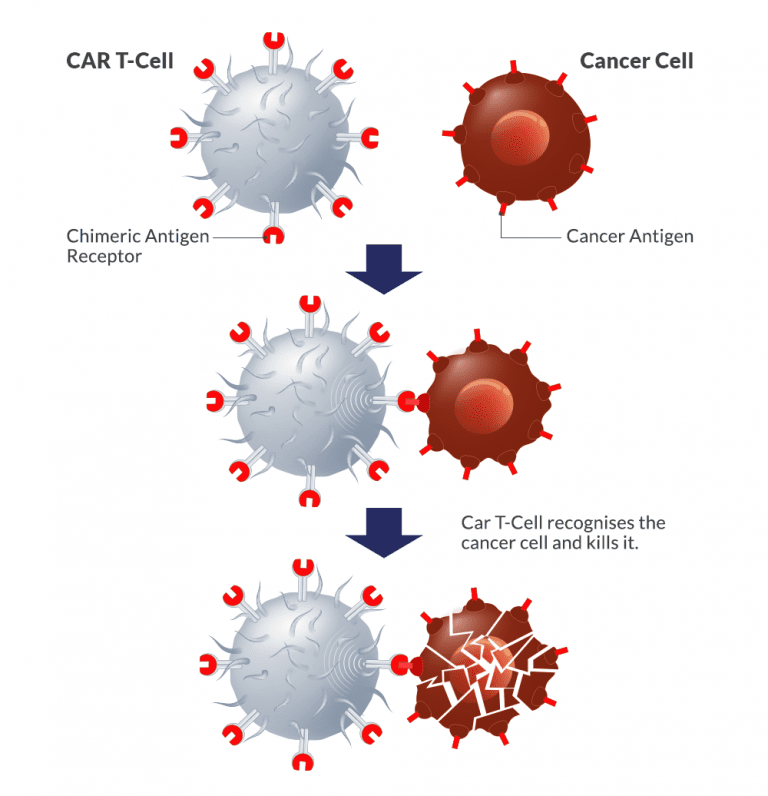
Right candidates for CAR T-Cell therapy
At present FDA has approved CAR T-Cell therapy for some forms of aggressive and refractory Non-Hodgkin lymphoma, Myeloma and relapsed and refractory acute lymphoblastic leukemia. Patient need to send full medical reports to ascertain the use of CAR T-Cell therapy for his treatment.
Inclusion criteria for CAR T-cell therapy :
1. Patients with CD19+ B-cell Lymphoma(At least 2 prior combination chemotherapy regimens)
2. To be aged 3 to 75 years
3. ECOG score ≤2
4. Women of childbearing potential must have a urine pregnancy test taken and proven negative prior to the treatment. All patients agree to use reliable methods of contraception during the trial period and until follow-up for the last time.
Exclusion criteria for CAR T-cell therapy :
1. Intracranial hypertension or unconsciousness
2. Respiratory failure
3. Disseminated intravascular coagulation
4. Hematosepsis or Uncontrolled active infection
5. Uncontrolled diabetes
Advantages of CAR T-Cell therapy
- >5000 CAR T cases done by highly skilled doctor’s.
- Hospitals in China has developed more CAR T Cell types including CD19 & CD 22 then any other country in the world.
- China is conducting more than 300 clinical trials on CAR T Cell therapy. More than any other country on the planet.
- Clinical effect of CAR T Cell is similar to that in USA or any other country and sometimes better.
Treatment process for CAR T-Cell therapy
- Complete evaluation of the patient
- T-cell collection from the body
- T-cells are then engineered in the lab
- Genetically engineered T-Cells are then multiplied by using growing them in the laboratory. These cells are frozen and then sent to the treating centres.
- Prior to infusing, patient may be given chemotherapy for their cancer. This helps the therapy work in a better way.
- Soon after chemotherapy CAR T-Cells are infused by a process which is similar to blood infusion.
- There is a 2-3 month of recovery period for the patient.
Time frame for CAR T-Cell therapy
1. Examination & test: one week
2. Pre-treatment & T-Cell Collection: one week
3. T-Cell preparation & return: two-three weeks
4. 1st Effectiveness analysis: three weeks
5. 2nd Effectiveness analysis: three weeks
Cost of CAR T-Cell therapy in Malaysia
Cost of CAR T-Cell therapy in Malaysia will be approximately between $ 45000 – 50,000 USD. For details on eligibility criteria and cost estimate please send your medical reports to info@cancerfax.com with your name and age as subject.
Also read this : CAR T Cell therapy in India
Side effects of CAR T-Cell therapy
The common side effects of CAR T-cell therapy include:
- Cytokine release syndrome
In some cases, patients may develop flu-like symptoms such as fever, chills, headache, nausea, vomiting, loose stools, and muscle or joint pains. It may also cause low blood pressure, difficulty in breathing, and a fast heart rate. These side effects are due to the release of cytokines by the immune cells during CAR T-cell therapy. These symptoms are usually mild, but can be serious and life threatening in some patients. - Neurological events
Neurological events can occur and can be serious in some patients. Such events include encephalopathy (brain injury and malfunction), confusion, difficulty speaking, agitation, seizures, drowsiness, altered state of consciousness and loss of balance. - Neutropaenia and Anaemia
Some patients may develop neutropenia or low white cell count. Similarly, anaemia or low red blood cell count may also occur due to this therapy.
Fortunately, most of these side effects usually resolve on their own or can be managed with the use of medications.
How effective is CAR T-Cell therapy ?
CAR T-cell therapy for the treatment of lymphoma and other blood cancers has shown promising outcomes. Since CAR T-cell treatment, many patients who had previously relapsed blood tumours had promising results and no evidence of cancer. It has also aided in the rehabilitation of patients who have previously failed to respond to most traditional cancer therapies.
However, longer-term studies for a larger patient population are needed to validate the efficacy of this treatment. Large-scale experiments would also aid in determining the likelihood of side effects and the right ways to deal with them.
Hospitals offering CAR T-cell therapy in Malaysia
- Sunway Medical Centre, Selangor
- Columbia Asia Hospital, Kuala Lumpur
How can I take treatment in Malaysia ?