LungVax: Lung cancer vaccine
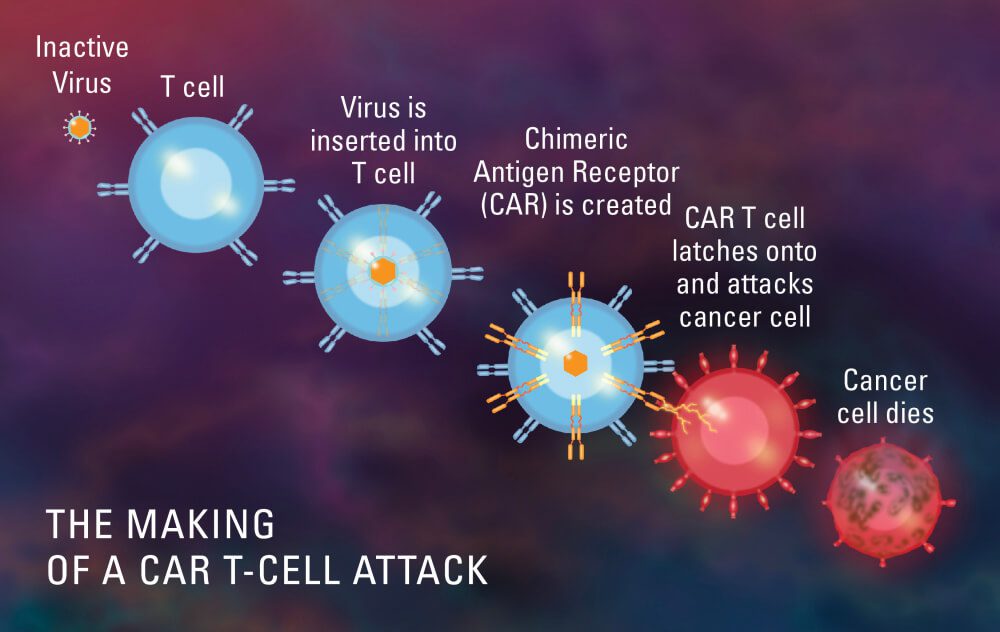
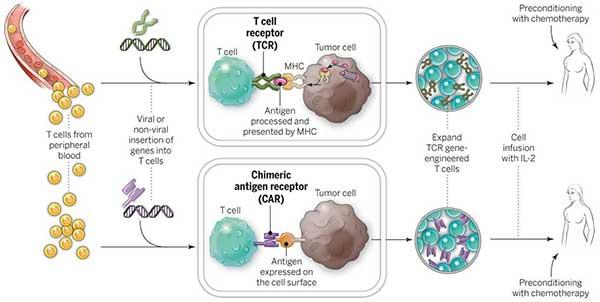
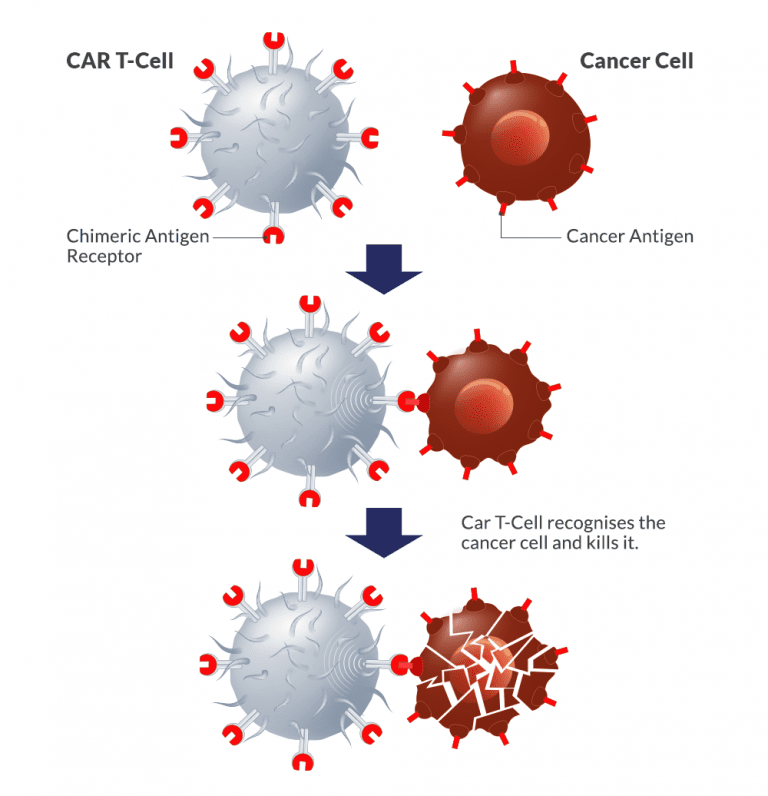
LungVax is an innovative lung cancer vaccine designed to stimulate the immune system to target and destroy cancer cells. It is engineered to enhance the body’s natural defense mechanisms against tumor growth, offering a novel approach to lung cancer treatment. LungVax aims to prevent recurrence in high-risk patients and improve survival rates, marking a promising development in immunotherapy for one of the deadliest forms of cancer.