
Gamma Delta T-Cell Therapy
In the last 25 years, T-cell treatments have made substantial progress in the treatment of cancer. Although much attention has been focused on chimeric antigen receptor T (CAR-T) cells and other cell therapies utilizing alpha beta (αβ) T cells, the gamma delta T (γδ T) cell, another subset of T cells, also shows great potential in fighting cancer.
Significant progress has been achieved in preclinical research on γδ T cells since their discovery in the 1980s. Various businesses, such as Cytomed, are developing γδ T-cell therapeutics.
γδ T cells possess distinct functional and therapeutic characteristics that set them apart from their more familiar αβ T cell counterparts, making them prospective candidates for cancer treatment.
Introduction
There exist two distinct categories of T cells: αβ T cells and γδ T cells. The previous statement indicates the presence of a T-cell receptor (TCR) that consists of a heterodimer composed of α and β chains. The latter refers to a T-cell receptor (TCR) that consists of a heterodimer made up of γ and δ chains.
These chains often do not have the co-receptors CD4 and CD8. On average, they make up around 4% of T cells found in human peripheral blood. Collectively, γδ T cells serve as a bridge between the innate and adaptive immune responses. Nevertheless, it is important to acknowledge that γδ T cells are not a uniform population, but rather a varied group of cells with distinct characteristics.
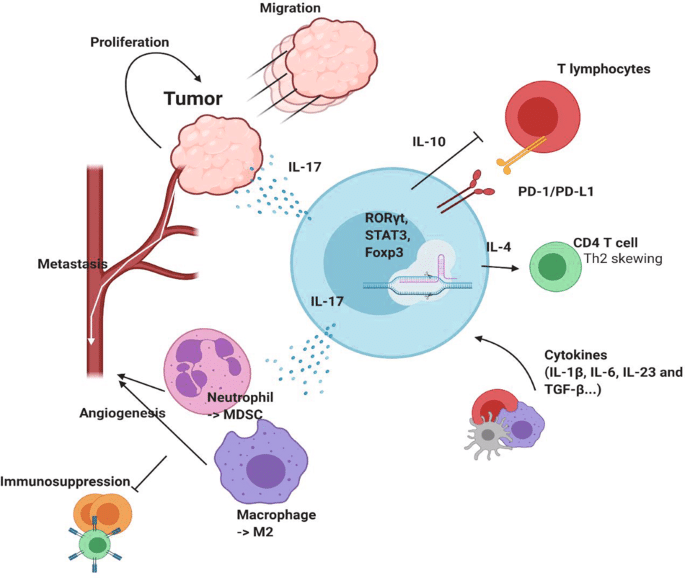
Human γδ T cells can be classified into two main groups, Vδ2 T cells and non-Vδ2 T cells, based on the expression of the TCR δ chain variable gene. The bulk of non-Vδ2 T cells are made up of γδ T cells that express Vδ1 and Vδ3. The distribution and frequency of the γδ T cell subset vary significantly between tissues and blood. Non-Vγ9Vδ2 T cells are now recognized as the primary subset of γδ T cells in various organs and lymphoid tissues, including the skin, colon, lungs, liver, lymph nodes, and thymus. These cells are believed to be adaptive-like γδ T cells.
The Vδ2 chain, when combined with a Vγ9 chain, is expressed in the majority (about 50%–95%) of γδ T cells seen in human blood. Vγ9Vδ2 T cells, a specialized subset, have the ability to universally detect phosphoantigens (PAgs) originating from microorganisms or altered cells. This recognition is achieved through the interaction with butyrophilin (BTN) family members BTN2A1 and BTN3A1. This fraction of T cells is considered innate-like γδ T cells due to their semi-invariant polyclonal expansion.
What and Where?
The thymus has a crucial role in the production of T lymphocytes, particularly in the early stages of development. The thymus, a tiny gland situated at the upper part of the sternum, serves as a breeding ground for T lymphocytes. It generates a variety of T lymphocytes that protect against infectious diseases or cancer.
The thymus houses undeveloped precursor T cells, known as thymocytes, which undergo maturation to become fully formed adult T cells, ultimately differentiating into many subtypes. They have the ability to produce well-established CD4 or CD8 T cells with recognized roles. These identical thymocytes also possess the potential to differentiate into γδ T cells and ultimately belong to one of the three primary subsets within this classification. The subsets are classified based on the specific type of delta chain (a component of the γδ T cell receptor) utilized, which consist of Vδ1, Vδ2, and Vδ3 cells.
Although delta chains exist in greater numbers, they are infrequent within the body and have received less research attention. American Gene Technologies’ primary emphasis for cancer therapy lies in the Vδ2 T cell, depicted in red, which is present in the blood, lymph nodes, and spleen.
Why are γδ T cells special?
T cells possess a diverse range of cell surface chemicals that serve to identify them and facilitate crucial cellular activities. Indeed, CD4, CD8, and γδ are three designations for distinct subsets of T cells that correspond to unique cell surface markers. In order to gain a deeper understanding of American Gene Technologies’ preferred γδ T cells, it is beneficial to be acquainted with the names of three crucial cell surface molecules that begin with γδ. These molecules are commonly referred to as the T cell receptor, NK receptor, and Fc receptor. Each possesses a distinct role that is crucial for the eradication of malignant cells.
The T cell receptor consists of two proteins, gamma and delta, which are responsible for the primary identification of these cells. The T cell receptor identifies antigens, which are stimulating compounds produced abundantly in tumor cells. The T cell receptor serves as both the ignition key and gas pedal for γδ T cell responses. It induces the proliferation of γδ T cells and enables them to carry out their primary activities, such as generating immune modulating chemicals (cytokines) and obtaining the capacity to eliminate cancerous cells. The NK receptor plays a crucial role in the exceptional capacity of γδ T cells to differentiate between tumor cells and normal cells.
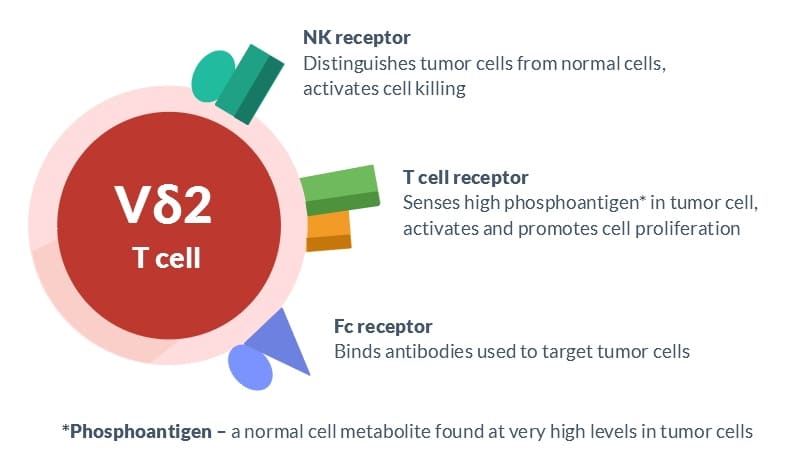
This receptor is a group of cell surface molecules that enhance the killing ability of γδ T lymphocytes when they come into contact with malignancies, while inhibiting this activity when they encounter normal cells. The Fc receptor is a molecular entity that specifically attaches to antibodies, especially those employed in cancer therapy. Simultaneously binding several antibodies can enhance the activation of γδ T lymphocytes, leading to increased tumor cell destruction.
Manufacturing process
Our CAR-γδ T cell technology differs from the traditional CAR-T cell technology by using healthy donor blood cells instead of patient blood cells. This allows us to create a standardized therapy called CTM-N2D that targets stress-induced cancer antigens. This therapy is suitable for a wide range of cancer patients.
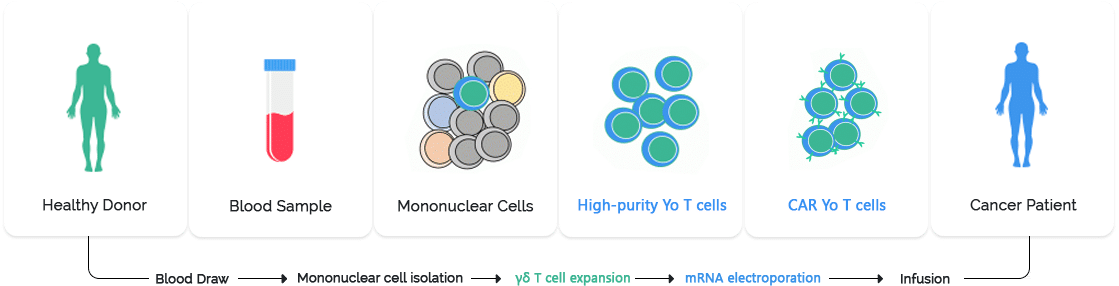
Pic: Manufacturing process of Gamma Delta T-Cell therapy
Gamma delta T cell therapy is an emerging form of immunotherapy that harnesses the unique properties of gamma delta (γδ) T cells to target cancer cells. The manufacturing process involves several critical steps to ensure the production of clinically effective and safe cell products.
Collection of Source Material: The process begins with the collection of peripheral blood mononuclear cells (PBMCs) from a healthy donor or the patient. This is typically done through leukapheresis, a procedure that isolates white blood cells from the blood.
Isolation of γδ T Cells: The collected PBMCs are then processed to isolate γδ T cells. This can be achieved through magnetic bead-based separation or flow cytometry techniques, which utilize specific markers to identify and select γδ T cells.
Activation and Expansion: Once isolated, γδ T cells are activated using stimulatory agents such as zoledronic acid or phosphoantigens. These agents promote the proliferation and activation of γδ T cells. The cells are then expanded ex vivo in a controlled environment using cytokines like IL-2 to achieve the necessary cell numbers for therapy.
Genetic Modification (if applicable): In some cases, γδ T cells are genetically modified to enhance their anti-tumor activity or to equip them with additional functions. This can involve viral or non-viral vector systems to introduce specific genes into the cells.
Quality Control and Testing: The expanded γδ T cells undergo rigorous quality control tests to ensure their safety, potency, and purity. This includes sterility testing, viability assessment, and functional assays to confirm their ability to target and kill cancer cells.
Cryopreservation and Storage: The final product is cryopreserved to maintain cell viability during storage and transportation. The cells are stored in a controlled environment until they are ready for administration to the patient.
Administration to Patient: The γδ T cell therapy is administered to the patient through an intravenous infusion. Post-infusion monitoring is essential to assess the therapy’s effectiveness and manage any potential side effects.
Mechanism of action
Gamma delta T cells play a crucial role in the innate immune response and are involved in immune surveillance. They can recognize and kill tumor cells directly through several mechanisms:
Direct cytotoxicity: γδ T cells can directly recognize and kill tumor cells. They release cytotoxic granules containing perforin and granzymes, leading to the induction of apoptosis in target cells.
Cytokine Production: These cells secrete various cytokines, such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which can inhibit tumor growth and recruit other immune cells to the tumor microenvironment.
Antigen-Presenting Cell (APC) Function: γδ T cells can act as antigen-presenting cells, enhancing the adaptive immune response by presenting tumor antigens to αβ T cells and other immune cells.
Stress Antigen Recognition: They recognize stress-induced molecules like MHC class I-related chain A (MICA) and B (MICB), which are often overexpressed on tumor cells.
Advantages of Gamma Delta T-cell therapy
Broad Target Recognition: Unlike αβ T cells, γδ T cells do not rely on MHC molecules for antigen recognition, allowing them to target a wide range of tumor antigens.
Rapid Response: As part of the innate immune system, γδ T cells can respond more rapidly to tumor cells compared to the adaptive immune response.
Minimal Risk of Graft-versus-Host Disease (GvHD): γδ T cells have a lower risk of causing GvHD, a significant complication in traditional T cell therapies, making them a safer option for allogeneic cell therapy.
Synergy with Other Therapies: γδ T cells can be used in combination with other treatments, such as checkpoint inhibitors and monoclonal antibodies, to enhance therapeutic efficacy.
Clinical application and research
The potential of γδ T cell therapy in cancer treatment is being explored in various clinical trials and preclinical studies. Some notable applications include:
Hematologic Malignancies: γδ T cells have shown promise in treating blood cancers such as leukemia and lymphoma. Their ability to recognize and kill malignant cells without MHC restriction makes them suitable for targeting these cancers.
Solid Tumors: Research is ongoing to harness γδ T cells in the treatment of solid tumors like breast, lung, and prostate cancers. The challenge lies in efficiently trafficking these cells to the tumor site and overcoming the immunosuppressive tumor microenvironment.
Combination Therapies: Combining γδ T cell therapy with other immunotherapies, such as checkpoint inhibitors, has demonstrated enhanced anti-tumor activity. This combinatorial approach leverages the strengths of both therapies to improve outcomes.
Challenges and future directions
Despite the promising potential, several challenges need to be addressed to fully realize the therapeutic benefits of γδ T cells:
Expansion and Activation: Efficiently expanding and activating γδ T cells ex vivo for therapeutic use is a critical challenge. Optimizing culture conditions and identifying suitable activation protocols are areas of active research.
Target Specificity and Homing: Ensuring that γδ T cells can specifically target tumor cells and effectively home to tumor sites is essential for therapeutic efficacy. Understanding the mechanisms underlying γδ T cell trafficking and tumor infiltration is crucial.
Tumor Microenvironment: The immunosuppressive tumor microenvironment poses a significant barrier to γδ T cell therapy. Strategies to modulate the tumor microenvironment and enhance the anti-tumor activity of γδ T cells are being investigated.
Clinical Trials and Safety: Rigorous clinical trials are necessary to evaluate the safety and efficacy of γδ T cell therapy in diverse cancer types. Monitoring for potential side effects and understanding the long-term outcomes of treatment are vital for clinical success.
Conclusion
Gamma delta T cell therapy represents a promising and innovative approach in the fight against cancer. Its unique properties, including broad antigen recognition, rapid response, and reduced risk of GvHD, position it as a potential game-changer in immunotherapy.
While challenges remain, ongoing research and clinical trials are paving the way for γδ T cells to become a cornerstone in cancer treatment. As our understanding of these remarkable cells deepens, their application could significantly improve outcomes for cancer patients, offering hope for more effective and targeted therapies in the future.