
CAR T-Cell Therapy in India
In October 2023, the Central Drugs Standard Control Organization (CDSCO), which is India’s equivalent of the US Food and Drug Administration, granted approval to NexCAR19, making it the first CAR-T cell therapy to be licensed in India. CAR T Cell therapy in India has been officially launched in 6 hospitals across Delhi, Mumbai, and Pune.
In the past few years, Indian hospitals and study centres have come a long way towards using CAR T-cell therapy. CAR T-cell therapy could change the way cancer is treated, so it gives patients who don’t have many other choices new hope. In this new treatment, the patient’s own immune cells are reprogrammed to find and kill cancer cells.
CAR T-Cell Therapy In India – Current Status
February, 2024: In October 2023, the Central Drugs Standard Control Organization (CDSCO), which is India’s equivalent of the US Food and Drug Administration, granted approval to NexCAR19, making it the first CAR-T cell therapy to be licensed in India. CAR T Cell therapy in India has been officially launched in 6 hospitals across Delhi, Mumbai, and Pune.
The license was granted based on the findings from two limited-scale clinical trials carried out in India involving a total of 64 individuals diagnosed with advanced lymphoma or leukemia. Based on the trial findings given at the American Society of Hematology meeting in December 2023, it was seen that 67% of the patients (36 out of 53) participating in the two studies experienced a significant reduction in the size of their cancer (objective response). Approximately half of these patients achieved total disappearance of the malignancy (complete response).
ImmunoACT, a subsidiary of IIT Bombay, has provided funding for the experiment and will be responsible for the production and commercialization of actalycabtagene autoleucel.
Apart from current ongoing clinical trials in ACTREC and Narayana, Bengaluru, MGRC has collaborated with a China-based CAR cell biotech company to bring CAR T-Cell therapy to India. At present, this life-saving therapy is available in the USA, UK, Canada, Israel, Singapore, China, Malaysia, & Australia. The cost of this therapy is around 5-7,00,000 USD in the USA, whereas in China it costs anywhere between $70,000 and $80,000 USD.

Clinical trials for CAR T- Cell therapy for the treatment of some types of blood cancer have kicked off at the Advanced Centre for Treatment, Research, and Education in Cancer, the research and development wing of Tata Memorial Centre. “More details of the trial will be revealed soon,” Dr. Narula said in a press brief. This clinical trial is taking place with the help of a researcher from IIT, Bombay, who has developed this life saving therapy.
Dr. Reddys lab has also secured a deal with Shenzen Biopharma Pregene of China on May 21 to bring this life-saving therapy to India. There are several other companies that are also working to bring this technology to India. US-based Indian-born oncologist Dr. Siddharth Mukherjee was in India recently and had a meeting with Kiran Mazumdar Shaw of Biocon & Mr. Kush Parmar of 5 AM Ventures. All of them have agreed to come up with a facility to grow a Chimeric Antigen Receptor (CAR) cells to fight cancer. As per the reports, this therapy can be available in India in about a year’s time. This therapy has recently been approved by FDA (Food and Drug Administration). This cell therapy is useful for treatment in certain children and young adults suffering from Non – Hodgkin lymphoma. Treatment with Yeskarta & Kymriah is the first CAR T-Cell therapy to receive FDA approval.
Despite the fact that there are multiple cell therapy clinical trials active and enrolling in the United States, the European Union, and China, none have been available in India.
The new Immuneel facility in Bengaluru’s Narayana Health City is dedicated to introducing high-quality and affordable cell therapies to India. The facility’s strategic location in a tertiary care hospital near a high-volume bone marrow transplant unit allows for further coordination between research teams and clinicians, which is important for focused clinical development of innovative personalized therapies like CAR-T.

Immuneel is working hard to advance its pipeline. The company’s strategy of licensing a CAR-T asset that has already been clinically tested is expected to result in the company’s first cell therapy clinical trial in 2021. In terms of laboratory and production facilities, including equipment and instruments, Immuneel’s integrated facility is among the best in the world. This helps physicians and scientists work together seamlessly both internally and with research institutes across the world on product creation and distribution. To support this target, the organization has attracted exceptional global talent with prior experience in cell therapy, as well as a distinguished Scientific Advisory Board consisting of the field’s most respected scientific and intellectual giants.
These therapies are labor-intensive, meticulously managed, require costly reagents and consumables, and are difficult to automate. The logistics of preserving and transporting cryopreserved cells continues to be a global problem. Because of all of these factors, cell therapies are exceedingly difficult to produce and supply and, thus, extremely costly. Cell therapies are difficult to prescribe clinically, and patients must be closely monitored for adverse events in the hospital immediately after infusion.
Result Of Phase II CAR T-Cell Therapy Clinical Trials In India
At ASCO, Dec 22 conference, Immuneel, a cell and gene therapy startup, announced that the preliminary findings from India’s first phase 2 clinical trials showed a 77% overall response rate at 90 days in patients with blood cancers like leukaemia and lymphoma. Immuneel is developing the CAR-T cell therapy Varnimcabtagene.
The initial findings from the IMAGINE trial were based on the enrollment of the first 10 individuals out of a total of 24 patients.
At day 28, over 80% of patients had a full clinical recovery. At Day 90, the IMAGINE data revealed an overall response rate of 77%, with complete responses being shown in 6 out of 9 evaluable patients.
The B acute lymphoblastic leukaemia patients’ day 28 and day 90 readouts show 100% and 83% complete remissions, respectively, indicating quick, strong, and long-lasting responses.
Varnimcabtagene took 12 days on average to produce and release, with a 100% manufacturing success rate.
Kiran Mazumdar-Shaw, chairwoman of Biocon, Siddhartha Mukherjee, a renowned oncologist and author, and Kush Parmar, managing partner of 5AM Venture, co-founded Imuneel. Imuneel is developing its own pipeline of chimeric antigen receptor T-cell (CAR-T) therapies and other cellular immunotherapies for the treatment of cancer.
What Is The Scope Of Car-T Cell Therapy In India?
Introduction: CAR T- cell therapy is a new type of treatment that is changing the way cancer is treated all over the world. In the past few years, India has made a lot of progress in adopting this cutting-edge treatment, giving patients with different kinds of cancer new reasons to hope. CAR-T cell therapy is likely to have a huge effect on Indian health care because it could change the way cancer is treated.
Expanding Treatment Options: The arrival of CAR-T cell therapy in India has given patients more ways to get treated, especially those with blood cancers like leukaemia and lymphoma. This therapy involves taking out a patient’s T cells, changing them genetically so that they produce chimeric antigen receptors (CARs) that target cancer cells, and then putting them back into the patient’s body. CAR-T cell therapy is a personalised method that makes it easier for the immune system to find and kill cancer cells.
Research and development: R&D has come a long way. India has a strong infrastructure for research and development, and top institutions and hospitals are actively looking into the potential of CAR-T cell therapy. This dedication to study has led to exciting new developments, such as the creation of CAR-T cell therapies that are tailored to the unique genetic and ethnic diversity of the Indian people. These kinds of improvements help to broaden the therapy’s reach and make it possible to use it on more types of cancer.
Affordability and accessibility: One of the best things about CAR-T cell treatment in India is that, compared to some Western countries, it is more affordable. Patients from all walks of life are more likely to be able to afford it because the prices are low and there are many treatment choices. Also, a number of Indian hospitals and medical centres have worked with international drug companies to bring CAR-T cell treatment to India, making it easier for people who need it to get it.
Challenges and Future Prospects: CAR-T cell treatment has a lot of potential, but it also has some problems. Some of the problems that need to be solved are the high cost of treatment, the difficulty of making the drug, and the need for specialised equipment. But the Indian government, working with healthcare stakeholders, is working hard to solve these problems and set up a regulatory system for the therapy to be used in a safe and effective way.
The use of CAR-T cell therapy in India is growing quickly, giving cancer patients new hope and better ways to treat their disease. India is a good place for this groundbreaking therapy because it is committed to study, prices are low, and access is getting better. As India’s health care system continues to change, the addition of CAR-T cell therapy is likely to change the way cancer is treated, making things better for patients and changing lives.
Where is CAR T Cell therapy available in India?
You can find CAR T-Cell therapy in several prominent Indian medical healthcare centers, such as Tata Memorial Centre, Apollo Cancer Hospital, BLK, Artemis, Asian Oncology, American Oncology, and HCG.
The availability of CAR T Cell Therapy in these prestigious healthcare facilities is a huge step in India’s fight against cancer. Find out the top 5 hospitals known for providing the best CAR-T treatment in India.
Tata Memorial Cancer Hospital In Mumbai
Among the leading providers of CAR T Treatment In India, first comes Tata Memorial Hospital, which is a world-class cancer treatment provider. In this hospital, a team of experienced doctors and researchers work hard to fight cancer using advanced treatments. The hospital is known for its excellence in cancer care and has a lot of experience in using CAR T Cell therapy to help patients get better. People come here from all over because they trust Tata Memorial to give them the best chance at beating cancer. So, if you or someone you know needs excellent cancer care, Tata Memorial Cancer Hospital is a great choice.
Apollo Cancer Institute In Chennai
The Apollo Cancer Institute in Chennai is a well-known healthcare center known for its excellent cancer treatment services. They provide comprehensive care to cancer patients through advanced technology and a highly experienced team of oncologists. Their dedicated team of oncologists, surgeons, and support staff work tirelessly to create personalized treatment plans that prioritize both effectiveness and the patient’s quality of life. Their commitment to excellence has earned them a respected reputation in the field of cancer care.
National Cancer Institute (AIIMS) In Delhi
The National Cancer Institute (AIIMS) in Delhi is another leading institution for CAR-T cell therapies. You can get access to CAR-T therapy here at the most affordable cost. This medical institute is well known for its advanced research on immunoadoptive cell therapy, top-notch facilities, and highly skilled doctors. The center takes a team approach, bringing together experienced oncologists, surgeons, radiologists, and support staff to create personalized treatment plans for blood cancer as well as all other types of cancer. They even use advanced technology like artificial intelligence and genetic analysis to provide the best cancer care to patients.
BLK Max Cancer Center, Delhi
The BLK Max Cancer Centre in Delhi is one of India’s leading cancer hospitals, dedicated to providing chimeric antigen receptor t cell therapy (CAR T). Their institute has advanced technology for cancer care, which includes robotic surgery, tomo therapy, and immunotherapy. Their warm and supportive environment can help you stay strong and combat the disease.
Rajiv Gandhi Cancer Institute & Research Centre In Delhi
The Rajiv Gandhi Cancer Institute & Research Centre in Delhi is one of Asia’s leading cancer centers. Their innovative technology and talented staff offer world-class cancer care for patients in India, as well as in SAARC nations. The institute has had the honor of positively influencing the lives of approximately 2.75 lakh cancer patients since its foundation in 1996. Their specialist offers the best possible care to cancer patients through cost-effective CAR T Cell therapy in India.
Meet The Top Oncologists For CAR T Cell therapy In India
Learn about the top oncologists for CAR T Treatment In India. These experienced doctors are committed to providing the best cell therapy for cancer along with personalized care and support. Trust in their expertise for a brighter future in your fight against cancer!
Dr T Raja (MD, DM)
Dr. T Raja is a well-known Medical Oncologist with over 25 years of experience in cancer treatment. He is well-known for his outstanding knowledge and is regarded as one of India’s top oncologists. Dr. Raja also holds a prominent academic position, leading the DNB Medical Oncology program at Apollo Specialty Hospital in Chennai. He is a sought-after speaker at national and international conferences, where he shares his valuable insights.
Dr Srikanth M (MD, DM)
Dr. Srikanth M. is a highly experienced hematologist in Chennai, known for his expertise in treating various blood-related conditions. He specializes in both short-term and long-term health issues like anemia, myeloma, b-cell lymphomas and leukaemia. Dr. Srikanth M. also provides advanced tests to check your overall health, such as bone marrow aspiration and chelation therapy for rare cases like an excess of minerals in the blood. Dr. Srikanth M. has received awards for his contributions to myeloma research, making him a trusted specialist in hematology ready to provide specialized care to patients in need.
Dr Revathi Raj (MD, DCH)
Dr. Revathi Raj is a highly respected specialist known for her expertise in pediatric bone marrow transplants. She has successfully conducted over 2000 of these transplants, making her India’s leading expert. Dr. Raj has extensive experience treating children with blood abnormalities such as thalassemia, hemophilia, sickle cell anemia, aplastic anemia, and leukemia. She is highly committed to the well-being of children, running a specialized service for pediatric leukemia and lymphoma with an 80% cure rate.
Cost Of Car T-Cell Treatment In India
On October 13, 2023, a company named Immunoadoptive Cell Therapy Private Limited (ImmunoACT) in Mumbai received permission from the Central Drugs Standard Control Organisation (CDSCO) for India’s first special cancer treatment called NexCAR19.
This treatment is specially designed to help people with certain types of leukemia and lymphoma who haven’t responded to other treatments. The clinical trial of this process was conducted on 60 patients with lymphomas and leukamia. The overall response rate of this pivotal clinical trial was 70% which marked it as a successful therapy to destroy cancer cells more effectively.
The cost of CAR T cell therapy in India is approximately USD 57,000. This price is much lower when compared to countries like the USA, Singapore, Malaysia etc. However, it’s crucial to note that this pricing can vary depending on a variety of factors. The CAR-T cell therapy costs might differ from one hospital to another depending on their technology, expertise, and other facilities.
Furthermore, the type of CAR T-cell therapy required and the condition of the patient may also affect the overall cost.
What Is Car T-Cell Therapy?
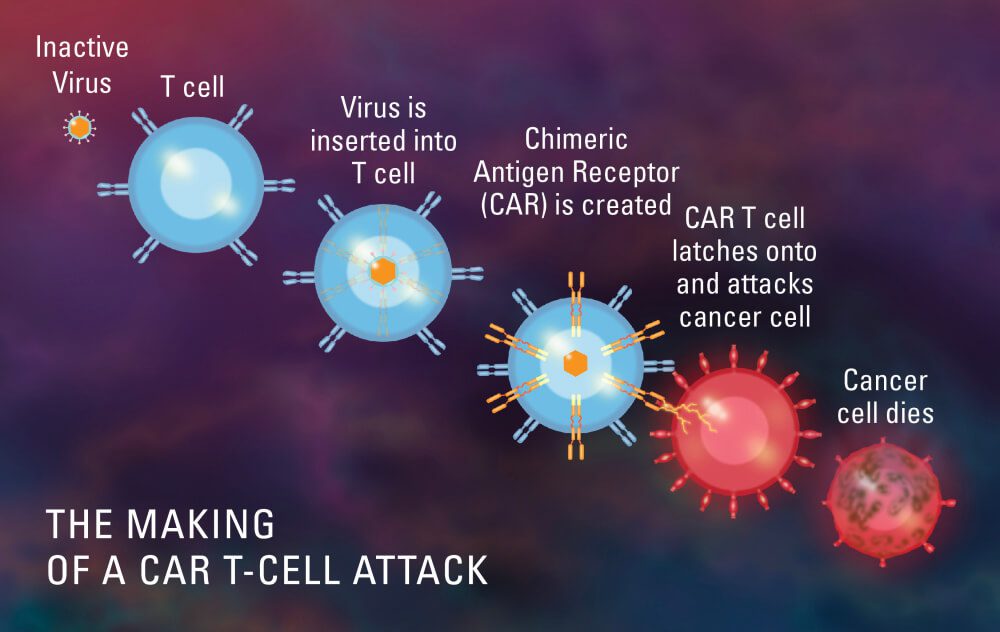
Chimeric antigen receptor T-cell therapy, often known as CAR T-cell therapy, is a ground-breaking immunotherapy that has completely changed the way that cancer is treated. It gives patients with certain cancers hope that were previously seen as incurable or with few therapeutic alternatives.
The treatment entails using a patient’s own immune cells—more specifically, T cells—and lab-modifying them to improve their capacity to detect and destroy cancer cells. To do this, the T cells are given a chimeric antigen receptor (CAR), which gives them the ability to target particular proteins, or antigens, on the surface of cancer cells.
T cells from the patient are first removed, and they are then genetically modified to express the CAR. In the laboratory, these altered cells are multiplied to produce a sizable population of CAR T cells, which are then put back into the patient’s bloodstream.
As soon as they are inside the body, CAR T cells find cancer cells that express the desired antigen, attach to them, and trigger a potent immune response. The CAR T cells that have been activated proliferate and conduct a focused attack on the cancer cells, killing them.
When used to treat some blood malignancies like acute lymphoblastic leukaemia (ALL) and specific forms of lymphoma, CAR T-cell therapy has shown exceptional results. It has produced notable response rates and in some patients, even long-lasting remissions.
CAR T-cell therapy, however, is a sophisticated and unique therapeutic method that might have risks and adverse effects. Cytokine release syndrome (CRS), a widespread immunological reaction that can result in flu-like symptoms and, in extreme situations, organ failure, may be experienced by certain people. There have also been reports of neurological adverse effects, however they are frequently curable.
Despite these difficulties, CAR T-cell therapy is a significant advancement in the fight against cancer and shows great potential for the future. Current studies are focused on enhancing its efficacy and safety profile as well as extending its uses to different cancer types. CAR T-cell therapy has the ability to change the face of cancer treatment and give patients everywhere new hope with further advancements.
Procedure
The CAR-T therapy procedure, which takes a few weeks, involves multiple steps:
T cells are extracted from your blood using a tube that is placed into an arm vein. This takes a couple of hours.
T cells are transported to a facility where they undergo genetic modification to become CAR-T cells. Two to three weeks pass throughout this.
CAR-T cells are reintroduced into your bloodstream through a drip. This requires several hours.
CAR-T cells target and eliminate cancer cells throughout the body. After receiving CAR-T therapy, you will be closely watched.
What Type Of Cancer Cells Can Be Treated With Car-T Cell Therapy?
Only patients with adult B-cell non-lymphoma Hodgkin’s or pediatric acute lymphoblastic leukemia who have already tried two unsuccessful conventional therapies can currently use CAR T-cell therapy products that have received FDA approval. However, CAR T-cell therapy is now being tested in clinical studies as a first or second-line treatment for adult lymphoma and pediatric acute lymphoblastic leukemia. Recently, some of the studies have shown remarkable successes in cases of solid tumors too, like glioblastoma, gliomas, liver cancer, lung cancer, GI cancer, pancreatic cancer, and oral cancer..
To Conclude
This represents a significant advancement in the management of leukemia and B-cell lymphoma. Additionally, it gives hope to those whose lives had previously been predicted to last only six months. Now that we have identified mechanisms of resistance and created more techniques to combat them, the future appears to be much more promising.
Get in touch with our highly experienced healthcare providers here at CancerFax for a free consultation to work out a suitable care plan for your healthcare needs. Please send your medical reports to info@cancerfax.com or WhatsApp to +1 213 789 56 55.
What Are The Advantages Of Car-T Cell Therapy?
The main benefit is that CAR T-cell therapy only requires a single infusion and often only requires two weeks of inpatient care. Patients with non-Hodgkin lymphoma and pediatric leukemia who have just been diagnosed, on the other hand, typically need chemotherapy for at least six months or more.
The advantages of CAR T-cell therapy, which is actually a living medication, can persist for many years. If and when a relapse occurs, the cells will still be able to identify and target cancer cells because they can survive in the body for an extended period of time.
Although the information is still developing, 42% of adult lymphoma patients who underwent CD19 CAR T-cell treatment were still in remission after 15 months. And after six months, two-thirds of patients with pediatric acute lymphoblastic leukemia were still in remission. Unfortunately, these patients had exceedingly aggressive tumors that weren’t successfully treated using traditional standards of care.
What type of patients would be good recipients of the CAR-T Cell Therapy?
Patients between the age of 3 Years to 70 Years have been tried with CAR T-Cell therapy for different type of blood cancers and has been found to be very effective. Many centers have claimed success rates of more than 80%. The optimum candidate for CAR T-cell therapy at this time is a juvenile with acute lymphoblastic leukemia or an adult with severe B-cell lymphoma who has already had two lines of ineffective therapy.
Before the end of 2017, there was no accepted standard of care for patients who had already gone through two lines of therapy without experiencing remission. The only FDA-approved treatment that has so far proven to be significantly beneficial for these patients is CAR T-cell therapy.
How Effective Is Car-T Cell Therapy?
CAR T-cell therapy has been very effective in treating some types of blood cancer, like acute lymphoblastic leukaemia (ALL) and non-Hodgkin lymphoma. In clinical trials, the response rates have been very good, and a lot of patients have gone into full remission. In some cases, people who had tried every other medicine had long-lasting remissions or even possible cures.
One of the best things about CAR T-cell treatment is that it targets the right cells. The CAR receptors that have been added to the T cells can find specific marks on cancer cells. This makes it possible to give targeted treatment. This targeted method hurts healthy cells as little as possible and lowers the risk of side effects that come with traditional treatments like chemotherapy.
But it’s important to keep in mind that CAR T-cell therapy is still a new area that is still changing. Researchers and doctors are working hard to solve problems like the high cost, the possibility of serious side effects, and the fact that it only works for some types of cancer.
In the end, CAR T-cell therapy has shown to be a very successful way to treat some types of blood cancer. Even though it is a promising and powerful method, more study and clinical trials are needed to improve it and find new ways to use it. CAR T-cell therapy could change how cancer is treated and make things better for people all over the world if it keeps getting better.
Inclusion & Exclusion Criteria
Inclusion Criteria For CAR T-Cell Therapy:
1. Patients with CD19+ B-cell Lymphoma(At least 2 prior combination chemotherapy regimens)
2. To be aged 3 to 75 years
3. ECOG score ≤2
4. Women of childbearing potential must have a urine pregnancy test taken and proven negative prior to the treatment. All patients agree to use reliable methods of contraception during the trial period and until follow-up for the last time.
Exclusion Criteria For CAR T-Cell Therapy:
1. Intracranial hypertension or unconsciousness
2. Respiratory failure
3. Disseminated intravascular coagulation
4. Hematosepsis or Uncontrolled active infection
5. Uncontrolled diabetes.
What Are The Side Effects Of Car-T Cell Therapy?
Below mentioned are some of the side-effects of CAR T-Cell therapy.
- Cytokine release syndrome (CRS): The most prevalent and possibly significant side effect of CAR T-cell treatment is cytokine release syndrome (CRS). The flu-like symptoms, including fever, exhaustion, headaches, and muscle pain, are brought on by the modified T cells’ production of cytokines. In extreme circumstances, CRS may result in a high temperature, hypotension, organ failure, and even potentially fatal consequences.
- Neurological Toxicity: Some patients may develop neurological side effects, which can range in severity from less serious signs like mild confusion and disorientation to more serious ones like seizures, delirium, and encephalopathy. After CAR T-cell infusion, neurological toxicity frequently happens during the first week.
- Cytopenias: CAR T-cell treatment can result in low blood cell counts, such as anaemia (low red blood cell count), neutropenia (low white blood cell count), and thrombocytopenia (low platelet count). Infections, bleeding, and exhaustion are among risks that can be exacerbated by these cytopenias.
- Infections: The CAR T-cell therapy’s suppression of healthy immune cells increases the risk of bacterial, viral, and fungal infections. In order to prevent infections, patients may need to be closely watched and given preventive medication.
- Tumour Lysis Syndrome (TLS): After CAR T-cell therapy, it’s possible in some circumstances for substantial amounts of cell contents to be released into the bloodstream due to the rapid killing of tumour cells. This may result in metabolic abnormalities, such as excessive potassium, uric acid, and phosphate levels, which may damage the kidneys and cause other problems.
- Hypogammaglobulinemia: CAR T-cell treatment has the potential to decrease antibody synthesis, which could result in hypogammaglobulinemia. This might make recurrent infections more likely and call for continuing antibody replacement medication.
- Organ Toxicity: CAR T-cell therapy has the potential to harm a number of organs, including the heart, lungs, liver, and kidneys. This may lead to abnormal renal function tests, respiratory issues, heart issues, and abnormal liver function tests.
- Hemophagocytic lymphohistiocytosis (HLH): A rare but possibly fatal immunological disease called hemophagocytic lymphohistiocytosis (HLH) can develop as a result of CAR T-cell therapy. It involves the overactivation of immune cells, which causes serious organ damage and inflammation.
- Hypotension and Fluid Retention: As a result of the cytokines that CAR T cells release, some patients may develop low blood pressure (hypotension) and fluid retention. To address these symptoms, supportive measures including intravenous fluids and drugs could be required.
- Secondary Malignancies: Reports of secondary malignancies emerging following CAR T-cell therapy exist, notwithstanding their rarity. Research is currently being done on the potential for secondary malignancies and long-term hazards.
It’s important to remember that not every patient will have these side effects, and that each person’s level of sensitivity will differ. In order to minimize and minimize these potential adverse effects, the medical team closely examines patients before, during, and after CAR T-cell therapy.
Time Frame
Check below the total time frame required for complete the CAR T-Cell therapy process. Although time frame depends a lot on the distance of lab from the hospital that prepared the CAR’s.
- Examination & test: one week
- Pre-treatment & T-Cell Collection: one week
- T-Cell preparation & return: two-three weeks
- 1st Effectiveness analysis: three weeks
- 2nd Effectiveness analysis: three weeks.
Total time frame: 10-12 Weeks
How We Can Help You Find The Best Cancer Treatment in India?
Finding the best cancer treatment in India can be quite difficult, especially if you’re worried about the expense as well as the quality. That’s where CancerFax can guide you like a true friend!
We are aware that your health is extremely valuable and that compromising on the quality of care is not an option. That’s why we’ve carefully selected highly experienced doctors and partnered with several hospitals at various pricing points so you may find the best fit for your needs. This way, you can receive excellent care without breaking the bank. For the last 10 years, our approach has already helped patients from more than 8 big countries, and we’re committed to doing the same for you. Trust us to receive the best CAR T Cell therapy in India.
Simplest Process Of Getting CAR T Cell Therapy In India
Send Your Reports
Share your medical history, including recent blood reports, biopsy results, and PET scans, with us at info@cancerfax.com. This crucial step allows us to assess your condition and guide you towards the most suitable treatment.
Evaluation & Opinion
Our team of experts will carefully review your reports to provide a thorough evaluation and expert opinion. This helps us determine the best course of action and recommend the most suitable hospitals and specialists for your CAR T Cell therapy as per your budget.
Medical Visa And Travel
We will help you in obtaining a medical visa and will plan your travel arrangements. Our objective is to make your trip as easy as possible so you can focus on your treatment and recovery.
Treatment And Follow Up
Once you arrive at the hospital of your choice, our devoted team will continue to help you throughout the treatment process. We promote seamless communication between you and the medical staff, ensuring you receive the best possible treatment. Your well-being remains our top priority.
FAQs On Car T-Cell Therapy In India
What is CAR T-cell therapy?
- CAR T-cell therapy is a type of immunotherapy that involves modifying a patient’s own T cells to recognize and attack cancer cells. This personalized treatment has shown promising results in certain types of cancer.
Is CAR T-cell therapy available in India?
- Yes, CAR T-cell therapy is available in India at some specialized cancer centers. However, its availability may vary, and it might be limited to certain types of cancers.
Which cancers can be treated with CAR T-cell therapy in India?
- As of my last update, CAR T-cell therapy was primarily used to treat certain types of blood cancers, such as leukemia and lymphoma. The specific cancers eligible for treatment may depend on the protocols followed by individual healthcare institutions.
What is the cost of CAR T-cell therapy in India?
- The cost of CAR T-cell therapy can be high. It may include the cost of collecting and modifying the patient’s T cells, the laboratory procedures, and the administration of the therapy. The cost can vary based on the type of cancer being treated and the healthcare facility.
Are there any side effects of CAR T-cell therapy?
- Yes, like any medical treatment, CAR T-cell therapy can have side effects. Common side effects include cytokine release syndrome (CRS) and neurologic toxicities. The severity of side effects can vary among individuals.
How successful is CAR T-cell therapy in treating cancer?
- CAR T-cell therapy has shown remarkable success in treating certain types of cancer, particularly blood cancers. However, its effectiveness can vary depending on the type and stage of cancer, as well as individual patient factors.
Is CAR T-cell therapy covered by insurance in India?
- Coverage by insurance may vary. It’s essential to check with the insurance provider and the healthcare facility offering the treatment to understand the coverage options.
How can I access CAR T-cell therapy in India?
- Patients interested in CAR T-cell therapy should consult with oncologists and healthcare providers who specialize in this treatment. They can guide patients through the evaluation process and determine eligibility.