Latest news in cancer
Mayo clinic trials triple negative breast cancer vaccine
Mayo Clinic Researchers at the Mayo Clinic Florida campus received a five-year federal grant totaling $ 13 million to test vaccines designed to prevent the...
First Global Oncology under MD Anderson Cancer Center provides consultation and referral services for cancer patients
MD Anderson Cancer Center is a global cancer diagnosis and treatment institution. It is a training hospital that countless domestic doctors aspire to. It is...
Brain metastasis in breast cancer
Breast cancer With the advancement of breast cancer diagnosis and treatment, the survival time of breast cancer patients has prolonged significantly, but the...
Targeted therapy for colorectal cancer
What are the colorectal cancer targeted drugs ? 17 years ago, the number of drugs available for advanced colorectal cancer was very limited. There were only a...
Recurrence of colorectal cancer
How to prevent recurrence of colorectal cancer ? How to treat recurrence of colorectal cancer after surgery ? Colorectal cancer is a common malignant tumor,...
Proton therapy for a ten-year-old girl with astrocytoma
Proton therapy in astrocytma was tried in 2012 for the first time. In 2012, Annabelle was diagnosed with fibroblastic astrocytoma, a brain tumor. Surgery...
CAR T-Cell therapy in solid tumors
July 2021: CAR T-Cell therapy in solid tumors has been approved in China with certain indications and markers. Recently, CAR T-Cell therapy is been tested for...
What is better for medulloblastoma – Traditional radiotherapy or proton therapy?
What is better for medulloblastoma - Traditional radiotherapy or proton therapy? Proton therapy for the treatment of medulloblastoma. Cost of proton therapy in...
Magic electric field for treatment of brain tumor
Magic electric field for treatment of brain tumor, the Best treatment of brain tumor in the world. Cost of magic electric field therapy in the treatment of...
New genetic test for childhood cancer
New genetic test for childhood cancer, 81 different genetic tests for childhood cancers. Genetic testing for childhood cancers in India. Cost of genetic...
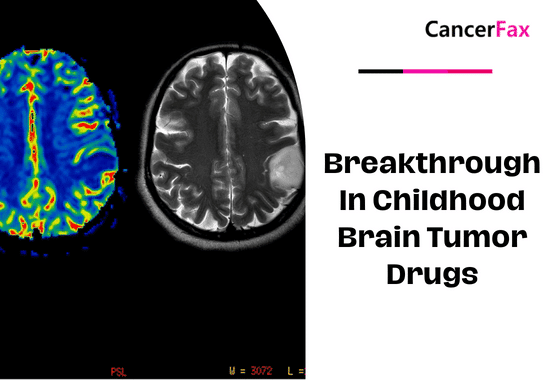
Breakthrough in childhood brain tumor drugs
Recent breakthroughs in childhood brain tumor drugs promise improved outcomes. Innovative therapies target specific genetic mutations, minimizing side effects...
CAR NK therapy has an effective rate of 73%
CAR NK therapy has an effective rate of 73% & is being recruited in clinical trials. CAR NK Cell therapy in India. Natural killer cell therapy in India. Cost...
First immunotherapy approved for esophageal cancer
First immunotherapy approved for esophageal cancer. Immunotherapy treatment in esophageal cancer treatment. Cost of immunotherapy in cancer treatment in...
8 major causes of kidney cancer
8 major causes of kidney cancer & how to avoid them ? How to diagnose kidney cancer. Kidney cancer signs, symptoms, diagnosis & best treatment options....
High cure rate for cervical cancer after proton therapy
High cure rate for cervical cancer after proton therapy. Effect of proton therapy in cervical cancer treatment. Complete cure for cervical cancer with proton...
Dietary problems for gastric cancer patients – How to manage?
Dietary problems for gastric cancer patients. How to manage food intake after stomach cancer surgery ? What to eat and what not to eat for stomach cancer...
Preventing recurrence of lung cancer
Preventing recurrence of lung cancer, how to prevent recurrence of lung cancer ? Preventing Recurrence of lung cancer, preventing recurrence after lung cancer...
CIMAvax and Vaxira – Vaccine for the treatment of non-small cell lung cancer
CIMAvax and Vaxira - Vaccine for the treatment of non-small cell lung cancer. Buy CIMAvax and Vaxira in India. Connect with +91 96 1588 1588 for buying lung...
Lung cancer vaccine after advanced lung cancer chemotherapy allows tumor to persist
Lung cancer vaccine after advanced lung cancer chemotherapy allows tumor to persist. Connect with us for best lung cancer treatment in India with best doctors...
No signs of tumor after proton therapy in an esophageal cancer patient
No signs of tumor after proton therapy in an esophageal cancer patient. Proton therapy in esophageal cancer patient who is 89 years of age. Patient can't be...
30+ Best Cancer Hospitals In The World 2024
Discover the best cancer hospitals in the world through our blog, which includes 30+ carefully selected facilities. We have picked top hospitals that use the...
Regular exercise reduces the risk of 7 different kinds of cancer
Regular exercise reduces the risk of 7 different kinds of cancer is been found out in a study conducted by American cancer society, The National Cancer...
Antioxidants may carry risk during breast cancer chemotherapy
Use of certain supplimnets like antioxidants may be risky during breast cancer chemotherapy. Cheap breast cancer chemotherapy drugs in India, economical breast...
CAR natural killer cell therapy – MD Anderson partners with Takeda
CAR Natural Killer-cell therapy. MD Anderson partners with Takeda to develop CAR NK cell therapy. CAR NK cell therapy is not yet available in India & hopefully...
CAR-NK therapy – New immunotherapy in cancer treatment
CAR-NK therapy is a new upcoming revolution in caner treatment. It's a type of immunotherapy for the treatment of some types of blood cancers. New developments...
Latest anticancer drugs in the market
Recent advancements in cancer treatment have led to the approval of several innovative drugs. For instance, the U.S. Food and Drug Administration (FDA) has...
Breakthrough in non-small cell lung cancer
The most recent development in non-small-cell lung cancer is a breakthrough. A vaccine with significant therapeutic impact on NSCLC has reached stage 3 of...
New treatment regimens for metastatic HER2-positive breast cancer
Metastatic HER2-positive breast cancer patients now have a potential new therapy choice. These choices have arisen as a consequence of two clinical trials that...
A drug used for alcoholism can treat cancer by targeting macrophages
Recent research has uncovered a promising avenue in cancer treatment by repurposing a drug originally used to combat alcoholism. This innovative approach...
Cancer cure – Latest immune discovery may cure cancer
In this article we discuss about a breakthrough immune discovery that holds promise for curing cancer. This new finding suggests a potential advancement in...
HIPEC Surgery in India
Top doctors, best hospitals & cost of HIPEC surgery in India. HIPEC surgery for GI cancer patients is best treatment. Call +91 96 1588 1588 for...
Effect of cancer treatment on heart
There are certain side effects of chemotherapy & radiotherapy on our heart in the treatment of cancer. Connect with +91 96 1588 1588 to know...
Treating brain tumor – A new approach to cancer treatment
Treating brain tumor in India is done with high level of expertise & new approach using latest technology by trained neurosurgeons. Connect with +91 96 1588...
New developments in the treatment of breast cancer
New developments in the treatment of breast cancer is well adopted in Indian hospitals by top oncologists. Stage 3 & stage 4 breast cancer treatment in India....
Dendritic cell based immunotherapy treatment in cancer
Dendritic cell based immunotherapy treatment in cancer in India. Connect with +91 96 1588 1588 for best alternative cancer treatment in...
Eating grapes may prevent you from cancer
Some research shows eating grapes may prevent cancer. Lung malignancy is the deadliest type of tumor on the planet, and 80% of deaths are associated with...
Need help? Our team is ready to assist you.
We wish a speedy recovery of your dear and near one.