Immuno-oncology is a way to treat cancer by using the body’s immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform that binds a patient’s own T cells to cancer cells. Because BiTE technology is flexible, it is easy to make molecules that attack tumor-specific antigens, which makes immuno-oncotherapy possible. Blinatumomab was the first standard BiTE molecule to be approved. It targets CD19 surface antigens on B cells and is mostly unaffected by genetic changes or escape mechanisms inside cells. More BiTE molecules are being made to treat other blood cancers (like multiple myeloma, acute myeloid leukaemia, and B-cell eitilæxli án Hodgkin) and solid tumours (like prostate cancer, glioblastoma, stomach cancer, and small-cell lung cancer). BiTE molecules that have a longer half-life than the standard ones are also being made. With BiTE technology, advances in immuno-oncology could make it easier to treat both blood and solid tumours and make them more effective when used with other treatments.
Hvað er BiTe meðferð?
Immuno-oncology therapies are scientifically proven ways to treat different types of solid and blóðkrabbamein. Hematologic cancers are a good fit for treatments that target the immune system because cancerous blood cells move around with immune cells. Several ónæmismeðferð meðferðir við krabbameini eru í vinnslu.
Monoclonal antibody checkpoint inhibitors that stop the binding of checkpoint proteins (like PD-1 and CTLA-4) are useful against many types of cancer. They work well and are safe for many solid tumours, especially when they target PD-1. Non-small-cell lung, kidney, and bladder cancers have all been treated successfully with these drugs. But many people don’t react to checkpoint inhibitors or get sick again after taking them. Except for non-Hodgkin eitilæxli, most results on hematologic cancers have been disappointing, especially for myeloma and leukaemia, where the overall response rate in approved indications ranges from 12.0% to 48.5%.8-15.
Other immuno-oncology treatments, on the other hand, have a higher success rate. Chimeric antigen-receptor (CAR) T-cell therapies change a patient’s T cells to attack a specific cellular antigen, such as CD19 in the treatment of B-cell malignancies and B-cell maturation antigen (BCMA) in the treatment of mörg mergæxli (MM). CAR T-cell treatments have shown promise in treating hematologic cancers. They haven’t been as effective in treating solid tumours, but there have been some good results with taugaæxli, human epidermal growth factor receptor tumours, and non-small-cell lung cancer. The genetic modification and in vitro multiplication of T cells take a long and complicated manufacturing process. This is a downside of this therapy because it makes it harder for patients to get this treatment quickly and in large numbers. The fact that lymphodepletion through chemotherapy preparation must be done first as a requirement for improved effectiveness is also a drawback.
BiTE (bispecific T-cell engager) meðferðir tengja eigin T frumur sjúklings við æxlistjáða mótefnavaka. Þetta kveikir á frumudrepandi getu eigin T-frumna sjúklingsins til að drepa krabbamein án þess að breyta genum T-frumnanna eða þurfa að vaxa eða meðhöndla þær utan líkamans. Hægt er að nota BiTE sameindir einar sér sem lyf eða með öðrum meðferðum til að gera þær skilvirkari.
BiTe verkunarháttur
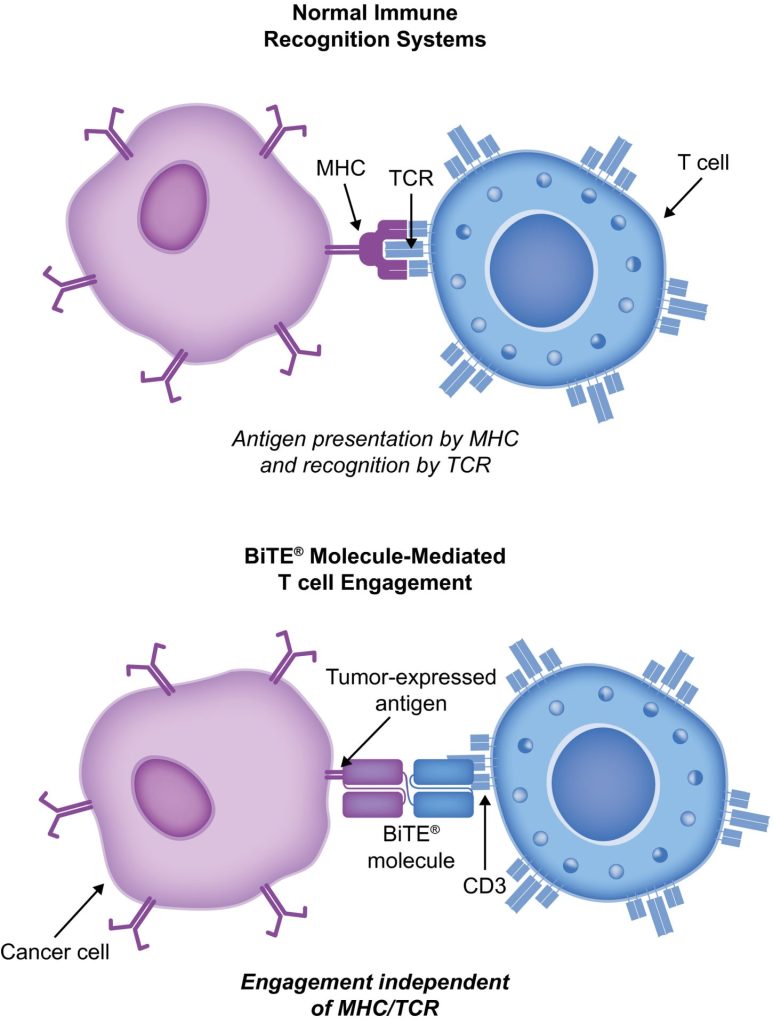
BiTE molecules are antibody constructs with two binding domains. One recognises tumor-expressed antigens (such as BCMA, CD19, or -like protein [DLL3]), and the other, CD3, recognises T cells (Fig. 1). Two single-chain variable fragment (scFv) regions from monoclonal antibodies are connected by a flexible peptide linker to make the binding domains. The first scFv binding region can be changed to target any surface antigen, so it can be used right away to treat a wide range of tumours and can be used again later. The second scFv binding region always binds to CD3, which is a part of the T-cell receptor complex that never changes. When a BiTE molecule interacts with both a cytotoxic T cell and a tumour cell, the T cells begin to multiply. This increases the amount of effector cells and makes BiTE therapy more effective. Then, the death of cancer cells is started. BiTE molecules can get any T cells to do this because they don’t need co-stimulation or the usual processes of the major histocompatibility complex.
Blinatumomab is the first and only BiTE therapy that has been approved. It targets the CD19 receptor on both normal and cancerous B cells. It is a highly potent molecule with cytotoxic effects seen at low exposures (10–100 pg/mL)26. In its presence, T cells can perform serial-target lysis, quickly binding to and killing many cells. This is how BiTE therapies work, and it can be seen in other BiTE molecules that are still in research. In bráð eitilfrumuhvítblæði (ALL), blinatumomab has been shown to be effective and safe. In 2014, the US Food and Drug Administration gave it fast approval, and in 2017, it got full approval for relapsed or refractory (R/R) B-cell precursor (BCP) ALL. In 2018, accelerated approval was given to blinatumomab for treating BCP-ALL with minimum residual disease (MRD). This was the first approval for this use. In November 2015, the European Medicines Agency also gave it a green light for BCP-ALL with a Philadelphia chromosome (Ph) that is negative and R/R. Blinatumomab is approved for R/R BCP-ALL in adults and children in 57 countries, including Japan, all countries in the European Union, Canada, and Australia.
Blinatumomab til meðferðar á sjúklingum með BCP-ALL
Blinatumomab hefur breytt því hvernig BCP-ALL er meðhöndlað. Í samanburði við staðlaða meðferð (SOC) krabbameinslyfjameðferð hefur hún aukið heildarlifun (OS) og dregið úr fjölda tiltekinna aukaverkana (AE). Nokkrar mikilvægar rannsóknir, þar á meðal slembiraðaðar samanburðarrannsóknir, sýndu að blinatumomab er öruggt og virkar fyrir BCP-ALL hjá bæði fullorðnum og börnum. C-T frumu meðferð, það eru aðeins gögn úr 2 einarma rannsóknum (clinicaltrials.gov auðkenni NCT01626495 og NCT01029366) þar sem 25 börn (5–22 ára) og 5 fullorðnir (26–60 ára) með R/R BCP-ALL og T-frumu ALL voru meðhöndluð. En niðurstöðurnar lofa góðu (algjör svörun [CR] hjá 90%, viðvarandi sjúkdómshlé með 6 mánaða lifun án atburða hjá 67% og heildarlifun [OS] hlutfall 78% [miðgildi eftirfylgni, 7 mánuðir; bil, 1-24 mánuðir]).
TOWER rannsóknin (A Phase 3, Randomized, Open Label Studigating the Efficacy of the BiTE Antibody Blinatumomab Versus Standard of Care Chemotherapy in Adult Subjects With Relapsed/Refractory B-Precursor ALL; clinicaltrials.gov auðkenni NCT02013167) bar saman áhrif PhOC-ealytherapy einlyfjameðferðar hjá fullorðnum við S heocrency. -neikvætt, R/R BCP-ALL. Þar sem fólk lifði lengur var náminu hætt snemma. Aukaverkanir í blinatumomab hópnum voru þær sömu og sáust í fyrri rannsóknum og blinatumomab var með lægri útsetningu leiðrétta aukaverkanatíðni en SOC.34 Blinatumomab virkar einnig fyrir fólk með Ph-jákvæð, R/R BCP-ALL og fyrir börn með Ph-neikvætt, R/R BCP-ALL.
30% to 50% of people with BCP-ALL in complete hematologic remission show persistent MRD. In the single-arm, phase 2 BLAST study (A Confirmatory Multicenter, Single-Arm Study to Assess the Efficacy, Safety, and Tolerability of the BiTE Antibody Blinatumomab in Adult Patients With MRD of B-Precursor Acute Lymphoblastic Leukaemia; clinicaltrials.gov identifier NCT01207388), blinatumomab was tested on patients with BCP-ALL in first or later complete After blinatumomab treatment, 78% of patients who were MRD positive became MRD negative. The 5-year OS study showed a median OS of 36.5 months, and more than half of those who had a complete MRD response after the first cycle of blinatumomab were still alive at 5 years, which suggests that the treatment might be able to cure some patients. AEs were seen that were linked to cýtókínlosunarheilkenni (CRS).31 Other studies, like NCT03023878 and NCT03340766, are still looking at blinatumomab in first-line settings and in combination with other treatments.
CD19-targeted treatments have been linked to failure because of the loss of CD19 antigen after treatment. The failure rates for blinatumomab range from 8% to 35%, and for CAR T-frumumeðferðir, they range from 39% to 65%.36-40 We don’t fully understand what causes therapy to fail, but one possibility is immunoediting, in which antigen loss is caused by a T-cell-dependent process called immunoselection, which lets tumour cells get away.41 Lineage switch and epitope loss under therapy pressure have also been suggested as ways for tumours to escape treatment. However, a recent study on epitope loss found that some CD19 isoforms that help CAR T-cells escape were already present at the time of diagnosis. This suggests that combining treatments might be helpful. Another thing that can cause immunotherapy to fail is called “inhibitory T-cell signalling.” In this case, the blocking programmed death ligand-1 (PD-L1) is interesting because it is more common in B-cell ALL cells from patients who don’t respond to blinatumomab and can make CD3 BiTE molecules less effective.43 By making a CD28/PD-L1 BiTE that triggers the CD28 co-stimulatory signal instead of the inhibitory signaling pathway that is usually seen when a T cell binds to a PD-L1-expressing cancer cell, this inhibition could be turned off.43 Dual-targeted CAR T cells are also being looked into as a way to make up for the loss of tumour antigens. This can be done by modifying each T cell with 2 CAR molecules and 2 different binding domains (dual-signaling CAR) or by putting 2 different binding domains on 1 CAR molecule at the same time (TanCAR).
Aukaverkanir með BiTE og stjórnun þess
Í klínískum rannsóknum á blinatumomab eru algengustu aukaverkanirnar hiti, lág fjöldi hvítra blóðkorna og lág blóðflagnafjöldi. Sumar mikilvægustu áhætturnar eru CRS, taugaeiturhrif og lyfjamistök. Taugaeiturhrif geta einnig gerst með CD19-sértækum CAR T-frumumeðferðum, en það gæti ekki verið vegna CD19. Niðurstöður áfanga 1/1b rannsókn sem er enn í gangi um CD20/CD3 markmið sýndu að miðtaugakerfisheilkenni af stigi 3 eða hærri voru sjaldgæf (3% af öllum gráðu 3 aukaverkunum). Oftast eru viðbrögð blinatumomabs við CRS væg, en í mjög sjaldgæfum tilfellum geta þau verið alvarleg og jafnvel lífshættuleg. Hægt er að draga úr bólguviðbrögðum með barksterum. Til að minnka líkur á CRS er best að gefa prednisón eða dexametasón innrennsli fyrir fyrsta skammtinn af blinatumomab og auka skammtinn hægt. Þessi notkun barkstera á undan öðrum BiTE sameindum hefur gefið tilefni til að nota dexametasón sem forlyf þegar aðrar BiTE sameindir eru notaðar. Hins vegar er ekki ljóst hvort hægt sé að beita þessum áhrifum á allan BiTE pallinn og verið er að skoða aðrar leiðir til að takast á við CRS. Interleukin 6 er cýtókín sem veldur CRS og er hátt í fólki sem hefur það. Tocilizumab, sem hindrar interleukin-6 viðtakann, hefur verið notað til að meðhöndla CRS sem er mjög slæmt eftir CAR T-frumumeðferð.49 Á sjúkrahúsinu hafa einnig verið notaðir æxlisdrep þáttahemlar til að meðhöndla CRS.


