Immuno-oncology is a way to treat cancer by using the body’s immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform that binds a patient’s own T cells to cancer cells. Because BiTE technology is flexible, it is easy to make molecules that attack tumor-specific antigens, which makes immuno-oncotherapy possible. Blinatumomab was the first standard BiTE molecule to be approved. It targets CD19 surface antigens on B cells and is mostly unaffected by genetic changes or escape mechanisms inside cells. More BiTE molecules are being made to treat other blood cancers (like multiple myeloma, acute myeloid leukaemia, and B-cell ne-Hodgkinov limfom) and solid tumours (like prostate cancer, glioblastoma, stomach cancer, and small-cell lung cancer). BiTE molecules that have a longer half-life than the standard ones are also being made. With BiTE technology, advances in immuno-oncology could make it easier to treat both blood and solid tumours and make them more effective when used with other treatments.
Što je BiTe terapija?
Immuno-oncology therapies are scientifically proven ways to treat different types of solid and karcinomi krvi. Hematologic cancers are a good fit for treatments that target the immune system because cancerous blood cells move around with immune cells. Several imunoterapija liječenje raka je u izradi.
Monoclonal antibody checkpoint inhibitors that stop the binding of checkpoint proteins (like PD-1 and CTLA-4) are useful against many types of cancer. They work well and are safe for many solid tumours, especially when they target PD-1. Non-small-cell lung, kidney, and bladder cancers have all been treated successfully with these drugs. But many people don’t react to checkpoint inhibitors or get sick again after taking them. Except for non-Hodgkin limfoma, most results on hematologic cancers have been disappointing, especially for myeloma and leukaemia, where the overall response rate in approved indications ranges from 12.0% to 48.5%.8-15.
Other immuno-oncology treatments, on the other hand, have a higher success rate. Chimeric antigen-receptor (CAR) T-cell therapies change a patient’s T cells to attack a specific cellular antigen, such as CD19 in the treatment of B-cell malignancies and B-cell maturation antigen (BCMA) in the treatment of multipli mijelom (MM). CAR T-cell treatments have shown promise in treating hematologic cancers. They haven’t been as effective in treating solid tumours, but there have been some good results with neuroblastom, human epidermal growth factor receptor tumours, and non-small-cell lung cancer. The genetic modification and in vitro multiplication of T cells take a long and complicated manufacturing process. This is a downside of this therapy because it makes it harder for patients to get this treatment quickly and in large numbers. The fact that lymphodepletion through chemotherapy preparation must be done first as a requirement for improved effectiveness is also a drawback.
Terapije BiTE (bispecifične T-stanice uključene) povezuju T-stanice pacijenta s antigenima izraženim u tumoru. Ovo uključuje citotoksičnu sposobnost pacijentovih vlastitih T stanica da ubiju rak bez mijenjanja gena T stanica ili potrebe za njihovim uzgojem ili manipulacijom izvan tijela. Molekule BiTE mogu se koristiti same kao lijekovi ili s drugim tretmanima kako bi bile učinkovitije.
BiTe mehanizam djelovanja
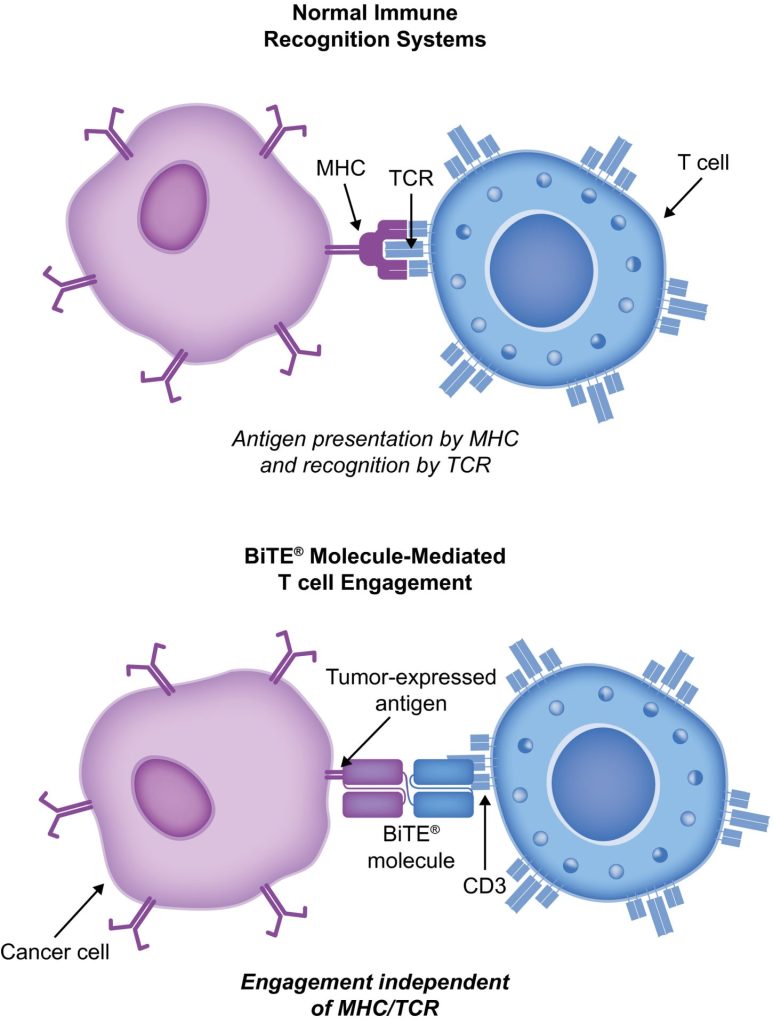
BiTE molecules are antibody constructs with two binding domains. One recognises tumor-expressed antigens (such as BCMA, CD19, or -like protein [DLL3]), and the other, CD3, recognises T cells (Fig. 1). Two single-chain variable fragment (scFv) regions from monoclonal antibodies are connected by a flexible peptide linker to make the binding domains. The first scFv binding region can be changed to target any surface antigen, so it can be used right away to treat a wide range of tumours and can be used again later. The second scFv binding region always binds to CD3, which is a part of the T-cell receptor complex that never changes. When a BiTE molecule interacts with both a cytotoxic T cell and a tumour cell, the T cells begin to multiply. This increases the amount of effector cells and makes BiTE therapy more effective. Then, the death of cancer cells is started. BiTE molecules can get any T cells to do this because they don’t need co-stimulation or the usual processes of the major histocompatibility complex.
Blinatumomab is the first and only BiTE therapy that has been approved. It targets the CD19 receptor on both normal and cancerous B cells. It is a highly potent molecule with cytotoxic effects seen at low exposures (10–100 pg/mL)26. In its presence, T cells can perform serial-target lysis, quickly binding to and killing many cells. This is how BiTE therapies work, and it can be seen in other BiTE molecules that are still in research. In akutna limfoblastična leukemija (ALL), blinatumomab has been shown to be effective and safe. In 2014, the US Food and Drug Administration gave it fast approval, and in 2017, it got full approval for relapsed or refractory (R/R) B-cell precursor (BCP) ALL. In 2018, accelerated approval was given to blinatumomab for treating BCP-ALL with minimum residual disease (MRD). This was the first approval for this use. In November 2015, the European Medicines Agency also gave it a green light for BCP-ALL with a Philadelphia chromosome (Ph) that is negative and R/R. Blinatumomab is approved for R/R BCP-ALL in adults and children in 57 countries, including Japan, all countries in the European Union, Canada, and Australia.
Blinatumomab za liječenje bolesnika s BCP-ALL
Blinatumomab je promijenio način na koji se liječi BCP-ALL. U usporedbi s kemoterapijom standardne skrbi (SOC), povećala je ukupno preživljenje (OS) i smanjila broj određenih nuspojava (AE). Nekoliko važnih studija, uključujući randomizirana kontrolirana ispitivanja, pokazalo je da je blinatumomab siguran i djeluje na BCP-ALL i kod odraslih i kod djece. Terapija T-stanicama CAR-a, postoje samo podaci iz 2 studije s jednim krakom (clinicaltrials.gov IDs NCT01626495 i NCT01029366) u kojima je liječeno 25 djece (dobi 5-22) i 5 odraslih (dobi 26-60) s R/R BCP-ALL i T-staničnim ALL-om. Ali rezultati su obećavajući (potpuni odgovor [CR] u 90%, održiva remisija sa 6-mjesečnim preživljenjem bez događaja u 67%, a ukupna stopa preživljenja [OS] od 78% [medijan praćenja, 7 mjeseci; raspon, 1-24 mjeseca]).
Studija TOWER (Faza 3, randomizirana, otvorena studija koja istražuje učinkovitost BiTE protutijela protiv blinatumomaba u odnosu na kemoterapiju standardne skrbi kod odraslih ispitanika s relapsom/refraktornim B-prekursorom ALL; Clinicaltrials.gov identifikator NCT02013167) uspoređivala je učinke monoterapije blinatumomabom u odnosu na SOC kemoterapiju kod odraslih koji su prethodno intenzivno liječeni. s Ph-negativnim, R/R BCP-ALL. Budući da su ljudi živjeli dulje, studija je rano prekinuta. Nuspojave u skupini liječenoj blinatumomabom bile su iste kao one viđene u ranijim studijama, a blinatumomab je imao niže stope nuspojava prilagođene izloženosti nego SOC.34 Blinatumomab također djeluje na ljude s Ph-pozitivnim, R/R BCP-ALL i za djecu s Ph-negativnim, R/R BCP-ALL.
30% to 50% of people with BCP-ALL in complete hematologic remission show persistent MRD. In the single-arm, phase 2 BLAST study (A Confirmatory Multicenter, Single-Arm Study to Assess the Efficacy, Safety, and Tolerability of the BiTE Antibody Blinatumomab in Adult Patients With MRD of B-Precursor Acute Lymphoblastic Leukaemia; clinicaltrials.gov identifier NCT01207388), blinatumomab was tested on patients with BCP-ALL in first or later complete After blinatumomab treatment, 78% of patients who were MRD positive became MRD negative. The 5-year OS study showed a median OS of 36.5 months, and more than half of those who had a complete MRD response after the first cycle of blinatumomab were still alive at 5 years, which suggests that the treatment might be able to cure some patients. AEs were seen that were linked to sindrom oslobađanja citokina (CRS).31 Other studies, like NCT03023878 and NCT03340766, are still looking at blinatumomab in first-line settings and in combination with other treatments.
CD19-targeted treatments have been linked to failure because of the loss of CD19 antigen after treatment. The failure rates for blinatumomab range from 8% to 35%, and for CAR T-stanične terapije, they range from 39% to 65%.36-40 We don’t fully understand what causes therapy to fail, but one possibility is immunoediting, in which antigen loss is caused by a T-cell-dependent process called immunoselection, which lets tumour cells get away.41 Lineage switch and epitope loss under therapy pressure have also been suggested as ways for tumours to escape treatment. However, a recent study on epitope loss found that some CD19 isoforms that help CAR T-cells escape were already present at the time of diagnosis. This suggests that combining treatments might be helpful. Another thing that can cause immunotherapy to fail is called “inhibitory T-cell signalling.” In this case, the blocking programmed death ligand-1 (PD-L1) is interesting because it is more common in B-cell ALL cells from patients who don’t respond to blinatumomab and can make CD3 BiTE molecules less effective.43 By making a CD28/PD-L1 BiTE that triggers the CD28 co-stimulatory signal instead of the inhibitory signaling pathway that is usually seen when a T cell binds to a PD-L1-expressing cancer cell, this inhibition could be turned off.43 Dual-targeted CAR T cells are also being looked into as a way to make up for the loss of tumour antigens. This can be done by modifying each T cell with 2 CAR molecules and 2 different binding domains (dual-signaling CAR) or by putting 2 different binding domains on 1 CAR molecule at the same time (TanCAR).
Nuspojave s BiTE-om i njegovo liječenje
U kliničkim studijama blinatumomaba, najčešći nuspojave su vrućica, nizak broj bijelih krvnih stanica i nizak broj trombocita. Neki od najvažnijih rizika su CRS, neurotoksičnost i pogreške u lijekovima. Neurotoksičnost se također može dogoditi kod tretmana CAR T-stanica specifičnih za CD19, ali možda nije zbog CD19. Rezultati studije faze 1/1b koja još uvijek traje o ciljevima CD20/CD3 pokazali su da su neželjeni događaji CNS-a stupnja 3 ili više rijetki (3% svih neželjenih događaja stupnja 3). Većinu vremena reakcija blinatumomaba na CRS je blaga, ali u rijetkim slučajevima može biti ozbiljna pa čak i opasna po život. Upalne reakcije mogu se umanjiti kortikosteroidima. Kako bi se smanjila mogućnost CRS-a, najbolje je dati infuziju prednizona ili deksametazona prije prve doze blinatumomaba i polagano povećavati dozu. Ova uporaba kortikosteroida prije drugih BiTE molekula dala je razlog za korištenje deksametazona kao premedikacije pri korištenju drugih BiTE molekula. Međutim, nije jasno može li se ovaj učinak primijeniti na cijelu BiTE platformu, a razmatraju se i drugi načini rješavanja CRS-a. Interleukin 6 je citokin koji uzrokuje CRS i visok je u ljudi koji ga imaju. Tocilizumab, koji blokira receptor interleukina-6, korišten je za liječenje CRS-a koji je vrlo loš nakon liječenja CAR T-stanicama.49 U bolnici su inhibitori faktora nekroze tumora također korišteni za liječenje CRS-a.


