Immuno-oncology is a way to treat cancer by using the body’s immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform that binds a patient’s own T cells to cancer cells. Because BiTE technology is flexible, it is easy to make molecules that attack tumor-specific antigens, which makes immuno-oncotherapy possible. Blinatumomab was the first standard BiTE molecule to be approved. It targets CD19 surface antigens on B cells and is mostly unaffected by genetic changes or escape mechanisms inside cells. More BiTE molecules are being made to treat other blood cancers (like multiple myeloma, acute myeloid leukaemia, and B-cell non-Hodgkinov lymfóm) and solid tumours (like prostate cancer, glioblastoma, stomach cancer, and small-cell lung cancer). BiTE molecules that have a longer half-life than the standard ones are also being made. With BiTE technology, advances in immuno-oncology could make it easier to treat both blood and solid tumours and make them more effective when used with other treatments.
Čo je BiTe terapia?
Immuno-oncology therapies are scientifically proven ways to treat different types of solid and rakoviny krvi. Hematologic cancers are a good fit for treatments that target the immune system because cancerous blood cells move around with immune cells. Several imunoterapia na liečbe rakoviny sa pracuje.
Monoclonal antibody checkpoint inhibitors that stop the binding of checkpoint proteins (like PD-1 and CTLA-4) are useful against many types of cancer. They work well and are safe for many solid tumours, especially when they target PD-1. Non-small-cell lung, kidney, and bladder cancers have all been treated successfully with these drugs. But many people don’t react to checkpoint inhibitors or get sick again after taking them. Except for non-Hodgkin lymfóm, most results on hematologic cancers have been disappointing, especially for myeloma and leukaemia, where the overall response rate in approved indications ranges from 12.0% to 48.5%.8-15.
Other immuno-oncology treatments, on the other hand, have a higher success rate. Chimeric antigen-receptor (CAR) T-cell therapies change a patient’s T cells to attack a specific cellular antigen, such as CD19 in the treatment of B-cell malignancies and B-cell maturation antigen (BCMA) in the treatment of mnohopočetný myelóm (MM). CAR T-cell treatments have shown promise in treating hematologic cancers. They haven’t been as effective in treating solid tumours, but there have been some good results with neuroblastómu, human epidermal growth factor receptor tumours, and non-small-cell lung cancer. The genetic modification and in vitro multiplication of T cells take a long and complicated manufacturing process. This is a downside of this therapy because it makes it harder for patients to get this treatment quickly and in large numbers. The fact that lymphodepletion through chemotherapy preparation must be done first as a requirement for improved effectiveness is also a drawback.
BiTE (bišpecifické zapojenie T-buniek) terapia spája pacientove vlastné T bunky s antigénmi exprimovanými v nádore. Tým sa zapne cytotoxická schopnosť pacientových vlastných T buniek zabíjať rakovinu bez toho, aby sa zmenili gény T buniek alebo museli rásť alebo manipulovať s nimi mimo tela. Molekuly BiTE môžu byť použité samostatne ako lieky alebo s inými liečbami, aby boli účinnejšie.
BiTe mechanizmus účinku
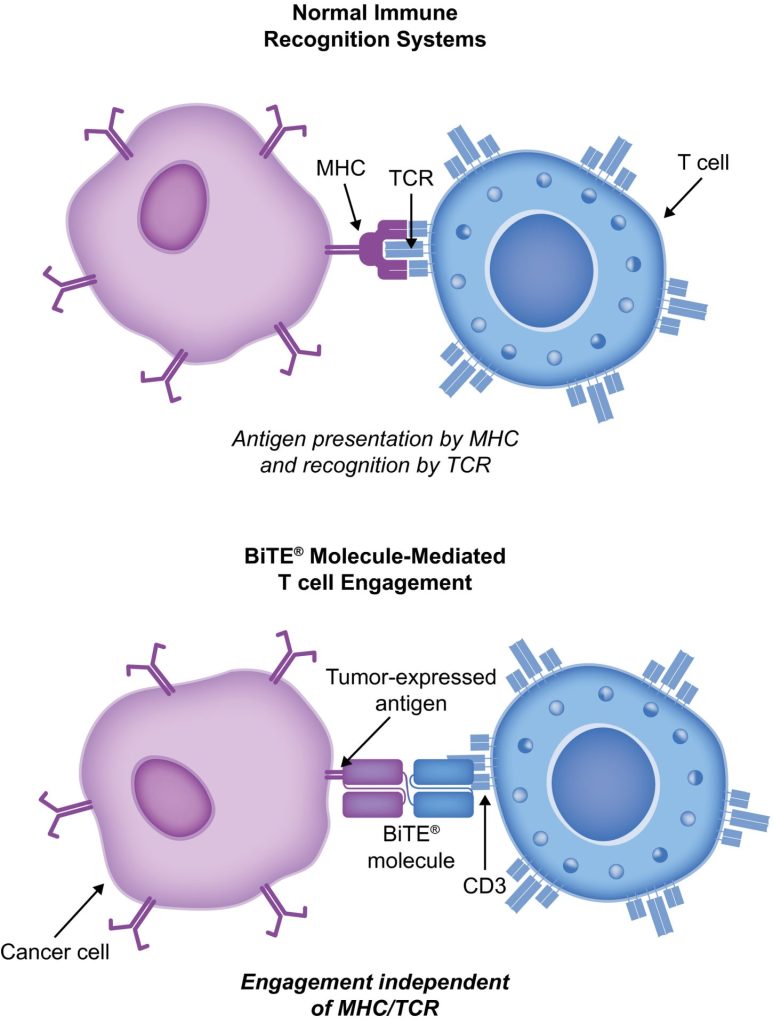
BiTE molecules are antibody constructs with two binding domains. One recognises tumor-expressed antigens (such as BCMA, CD19, or -like protein [DLL3]), and the other, CD3, recognises T cells (Fig. 1). Two single-chain variable fragment (scFv) regions from monoclonal antibodies are connected by a flexible peptide linker to make the binding domains. The first scFv binding region can be changed to target any surface antigen, so it can be used right away to treat a wide range of tumours and can be used again later. The second scFv binding region always binds to CD3, which is a part of the T-cell receptor complex that never changes. When a BiTE molecule interacts with both a cytotoxic T cell and a tumour cell, the T cells begin to multiply. This increases the amount of effector cells and makes BiTE therapy more effective. Then, the death of cancer cells is started. BiTE molecules can get any T cells to do this because they don’t need co-stimulation or the usual processes of the major histocompatibility complex.
Blinatumomab is the first and only BiTE therapy that has been approved. It targets the CD19 receptor on both normal and cancerous B cells. It is a highly potent molecule with cytotoxic effects seen at low exposures (10–100 pg/mL)26. In its presence, T cells can perform serial-target lysis, quickly binding to and killing many cells. This is how BiTE therapies work, and it can be seen in other BiTE molecules that are still in research. In akútna lymfoblastická leukémia (ALL), blinatumomab has been shown to be effective and safe. In 2014, the US Food and Drug Administration gave it fast approval, and in 2017, it got full approval for relapsed or refractory (R/R) B-cell precursor (BCP) ALL. In 2018, accelerated approval was given to blinatumomab for treating BCP-ALL with minimum residual disease (MRD). This was the first approval for this use. In November 2015, the European Medicines Agency also gave it a green light for BCP-ALL with a Philadelphia chromosome (Ph) that is negative and R/R. Blinatumomab is approved for R/R BCP-ALL in adults and children in 57 countries, including Japan, all countries in the European Union, Canada, and Australia.
Blinatumomab na liečbu pacientov s BCP-ALL
Blinatumomab zmenil spôsob liečby BCP-ALL. V porovnaní s chemoterapiou štandardnej starostlivosti (SOC) zvýšila celkové prežitie (OS) a znížila počet určitých vedľajších účinkov (AE). Niekoľko dôležitých štúdií vrátane randomizovaných kontrolovaných štúdií ukázalo, že blinatumomab je bezpečný a funguje pri BCP-ALL u dospelých aj detí. Liečba T-bunkami CAR, existujú iba údaje z 2 jednoramenných štúdií (clinicaltrials.gov ID NCT01626495 a NCT01029366), v ktorých 25 detí (vo veku 5 – 22 rokov) a 5 dospelých (vo veku 26 – 60 rokov) s R/R BCP-ALL a T-bunkami VŠETCI boli liečení. Výsledky sú však sľubné (úplná odpoveď [CR] u 90 %, trvalá remisia so 6-mesačným prežívaním bez príhody u 67 % a miera celkového prežívania [OS] 78 % [medián sledovania, 7 mesiacov; rozsah, 1 – 24 mesiacov]).
Štúdia TOWER (fáza 3, randomizovaná, otvorená štúdia skúmajúca účinnosť protilátky BiTE Blinatumomab oproti štandardnej chemoterapii starostlivosti u dospelých pacientov s ALL s relapsom/refraktérnym B-prekurzorom ALL; identifikátor Clinictrials.gov NCT02013167) porovnávala účinky monoterapie blinatumomab proti chemoterapii SOC u intenzívne predliečených dospelých s Ph-negatívnym, R/R BCP-ALL. Keďže ľudia žili dlhšie, štúdia bola predčasne zastavená. AE v skupine s blinatumomabom boli rovnaké ako tie, ktoré sa pozorovali v skorších štúdiách a blinatumomab mal nižšie miery AE upravených na expozíciu ako SOC.34 Blinatumomab funguje aj u ľudí s Ph-pozitívnym, R/R BCP-ALL a pre deti s Ph- negatívny, R/R BCP-ALL.
30% to 50% of people with BCP-ALL in complete hematologic remission show persistent MRD. In the single-arm, phase 2 BLAST study (A Confirmatory Multicenter, Single-Arm Study to Assess the Efficacy, Safety, and Tolerability of the BiTE Antibody Blinatumomab in Adult Patients With MRD of B-Precursor Acute Lymphoblastic Leukaemia; clinicaltrials.gov identifier NCT01207388), blinatumomab was tested on patients with BCP-ALL in first or later complete After blinatumomab treatment, 78% of patients who were MRD positive became MRD negative. The 5-year OS study showed a median OS of 36.5 months, and more than half of those who had a complete MRD response after the first cycle of blinatumomab were still alive at 5 years, which suggests that the treatment might be able to cure some patients. AEs were seen that were linked to syndróm uvoľnenia cytokínov (CRS).31 Other studies, like NCT03023878 and NCT03340766, are still looking at blinatumomab in first-line settings and in combination with other treatments.
CD19-targeted treatments have been linked to failure because of the loss of CD19 antigen after treatment. The failure rates for blinatumomab range from 8% to 35%, and for CAR T-bunkové terapie, they range from 39% to 65%.36-40 We don’t fully understand what causes therapy to fail, but one possibility is immunoediting, in which antigen loss is caused by a T-cell-dependent process called immunoselection, which lets tumour cells get away.41 Lineage switch and epitope loss under therapy pressure have also been suggested as ways for tumours to escape treatment. However, a recent study on epitope loss found that some CD19 isoforms that help CAR T-cells escape were already present at the time of diagnosis. This suggests that combining treatments might be helpful. Another thing that can cause immunotherapy to fail is called “inhibitory T-cell signalling.” In this case, the blocking programmed death ligand-1 (PD-L1) is interesting because it is more common in B-cell ALL cells from patients who don’t respond to blinatumomab and can make CD3 BiTE molecules less effective.43 By making a CD28/PD-L1 BiTE that triggers the CD28 co-stimulatory signal instead of the inhibitory signaling pathway that is usually seen when a T cell binds to a PD-L1-expressing cancer cell, this inhibition could be turned off.43 Dual-targeted CAR T cells are also being looked into as a way to make up for the loss of tumour antigens. This can be done by modifying each T cell with 2 CAR molecules and 2 different binding domains (dual-signaling CAR) or by putting 2 different binding domains on 1 CAR molecule at the same time (TanCAR).
Nežiaduce udalosti s BiTE a jeho manažmentom
V klinických štúdiách blinatumomabu sú najčastejšími AE horúčka, nízky počet bielych krviniek a nízky počet krvných doštičiek. Niektoré z najdôležitejších rizík sú CRS, neurotoxicita a drogové chyby. Neurotoxicita sa môže vyskytnúť aj pri liečbe CAR T-bunkami špecifickými pre CD19, ale nemusí to byť spôsobené CD19. Výsledky štúdie fázy 1/1b, ktorá stále prebieha o cieľoch CD20/CD3, ukázali, že nežiaduce účinky CNS 3. alebo vyššieho stupňa boli zriedkavé (3 % všetkých nežiaducich účinkov 3. stupňa). Reakcia blinatumomabu na CRS je väčšinou mierna, ale v zriedkavých prípadoch môže byť závažná a dokonca život ohrozujúca. Zápalové reakcie možno zmierniť kortikosteroidmi. Na zníženie pravdepodobnosti CRS je najlepšie podať infúziu prednizónu alebo dexametazónu pred prvou dávkou blinatumomabu a dávku pomaly zvyšovať. Toto použitie kortikosteroidov pred inými molekulami BiTE poskytlo dôvod na použitie dexametazónu ako premedikácie pri použití iných molekúl BiTE. Nie je však jasné, či sa tento efekt dá aplikovať na celú platformu BiTE a skúmajú sa aj iné spôsoby riešenia CRS. Interleukín 6 je cytokín, ktorý spôsobuje CRS a je vysoký u ľudí, ktorí ho majú. Tocilizumab, ktorý blokuje receptor interleukínu-6, sa používa na liečbu CRS, ktorá je veľmi zlá po liečbe CAR T-bunkami.49 V nemocnici sa na liečbu CRS používajú aj inhibítory tumor nekrotizujúceho faktora.


