Immuno-oncology is a way to treat cancer by using the body’s immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform that binds a patient’s own T cells to cancer cells. Because BiTE technology is flexible, it is easy to make molecules that attack tumor-specific antigens, which makes immuno-oncotherapy possible. Blinatumomab was the first standard BiTE molecule to be approved. It targets CD19 surface antigens on B cells and is mostly unaffected by genetic changes or escape mechanisms inside cells. More BiTE molecules are being made to treat other blood cancers (like multiple myeloma, acute myeloid leukaemia, and B-cell ikke-Hodgkin-lymfom) and solid tumours (like prostate cancer, glioblastoma, stomach cancer, and small-cell lung cancer). BiTE molecules that have a longer half-life than the standard ones are also being made. With BiTE technology, advances in immuno-oncology could make it easier to treat both blood and solid tumours and make them more effective when used with other treatments.
Hva er BiTe-terapi?
Immuno-oncology therapies are scientifically proven ways to treat different types of solid and blodkreft. Hematologic cancers are a good fit for treatments that target the immune system because cancerous blood cells move around with immune cells. Several immunterapi behandlinger for kreft er i arbeid.
Monoclonal antibody checkpoint inhibitors that stop the binding of checkpoint proteins (like PD-1 and CTLA-4) are useful against many types of cancer. They work well and are safe for many solid tumours, especially when they target PD-1. Non-small-cell lung, kidney, and bladder cancers have all been treated successfully with these drugs. But many people don’t react to checkpoint inhibitors or get sick again after taking them. Except for non-Hodgkin lymfom, most results on hematologic cancers have been disappointing, especially for myeloma and leukaemia, where the overall response rate in approved indications ranges from 12.0% to 48.5%.8-15.
Other immuno-oncology treatments, on the other hand, have a higher success rate. Chimeric antigen-receptor (CAR) T-cell therapies change a patient’s T cells to attack a specific cellular antigen, such as CD19 in the treatment of B-cell malignancies and B-cell maturation antigen (BCMA) in the treatment of flere myelomer (MM). CAR T-cell treatments have shown promise in treating hematologic cancers. They haven’t been as effective in treating solid tumours, but there have been some good results with neuroblastom, human epidermal growth factor receptor tumours, and non-small-cell lung cancer. The genetic modification and in vitro multiplication of T cells take a long and complicated manufacturing process. This is a downside of this therapy because it makes it harder for patients to get this treatment quickly and in large numbers. The fact that lymphodepletion through chemotherapy preparation must be done first as a requirement for improved effectiveness is also a drawback.
BiTE-behandlinger (bispecific T-cell Engager) kobler en pasients egne T-celler til tumoruttrykte antigener. Dette slår på den cytotoksiske evnen til pasientens egne T-celler til å drepe kreft uten å endre T-cellenes gener eller trenger å vokse eller manipulere dem utenfor kroppen. BiTE-molekyler kan brukes alene som medisiner eller sammen med andre behandlinger for å gjøre dem mer effektive.
BiTe virkningsmekanisme
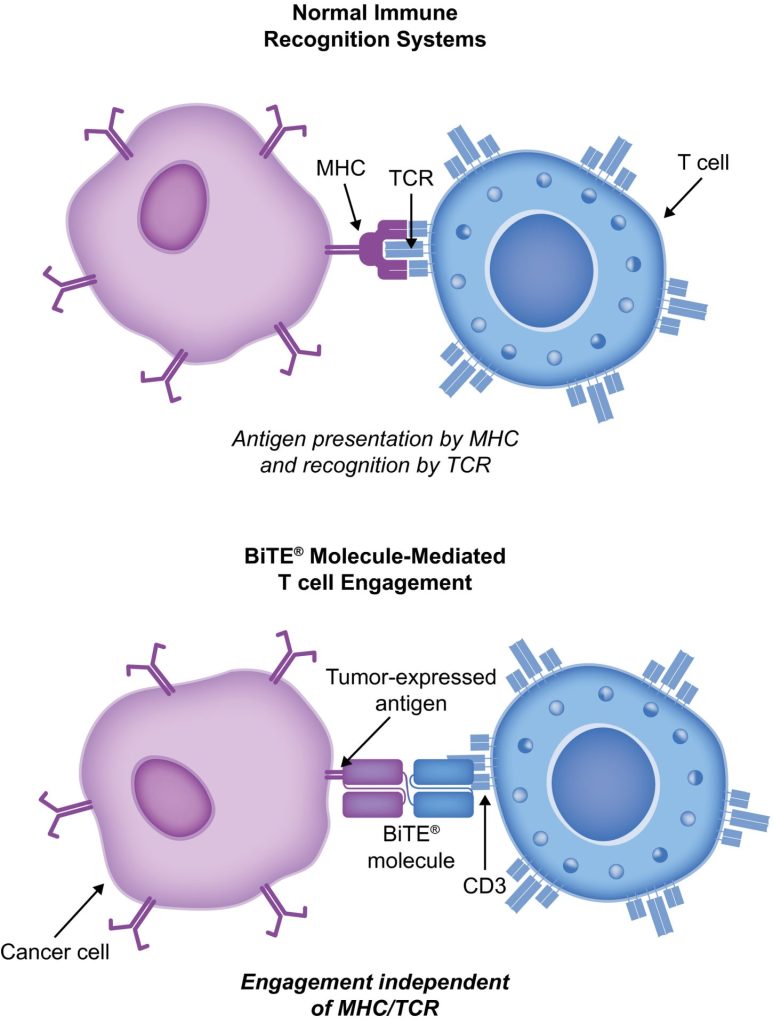
BiTE molecules are antibody constructs with two binding domains. One recognises tumor-expressed antigens (such as BCMA, CD19, or -like protein [DLL3]), and the other, CD3, recognises T cells (Fig. 1). Two single-chain variable fragment (scFv) regions from monoclonal antibodies are connected by a flexible peptide linker to make the binding domains. The first scFv binding region can be changed to target any surface antigen, so it can be used right away to treat a wide range of tumours and can be used again later. The second scFv binding region always binds to CD3, which is a part of the T-cell receptor complex that never changes. When a BiTE molecule interacts with both a cytotoxic T cell and a tumour cell, the T cells begin to multiply. This increases the amount of effector cells and makes BiTE therapy more effective. Then, the death of cancer cells is started. BiTE molecules can get any T cells to do this because they don’t need co-stimulation or the usual processes of the major histocompatibility complex.
Blinatumomab is the first and only BiTE therapy that has been approved. It targets the CD19 receptor on both normal and cancerous B cells. It is a highly potent molecule with cytotoxic effects seen at low exposures (10–100 pg/mL)26. In its presence, T cells can perform serial-target lysis, quickly binding to and killing many cells. This is how BiTE therapies work, and it can be seen in other BiTE molecules that are still in research. In akutt lymfatisk leukemi (ALL), blinatumomab has been shown to be effective and safe. In 2014, the US Food and Drug Administration gave it fast approval, and in 2017, it got full approval for relapsed or refractory (R/R) B-cell precursor (BCP) ALL. In 2018, accelerated approval was given to blinatumomab for treating BCP-ALL with minimum residual disease (MRD). This was the first approval for this use. In November 2015, the European Medicines Agency also gave it a green light for BCP-ALL with a Philadelphia chromosome (Ph) that is negative and R/R. Blinatumomab is approved for R/R BCP-ALL in adults and children in 57 countries, including Japan, all countries in the European Union, Canada, and Australia.
Blinatumomab for behandling av pasienter med BCP-ALL
Blinatumomab har endret måten BCP-ALL behandles på. Sammenlignet med standard-of-care (SOC) kjemoterapi, har det økt total overlevelse (OS) og redusert antall visse bivirkninger (AE). Flere viktige studier, inkludert randomiserte kontrollerte studier, viste at blinatumomab er trygt og virker for BCP-ALL hos både voksne og barn. C-T-cellebehandling, er det kun data fra 2 enarmsstudier (clinicaltrials.gov IDs NCT01626495 og NCT01029366) der 25 barn (alder 5–22) og 5 voksne (alder 26–60) med R/R BCP-ALL og T-celle ALL ble behandlet. Men resultatene er lovende (en fullstendig respons [CR] hos 90 %, vedvarende remisjon med 6 måneders hendelsesfri overlevelse hos 67 %, og en total overlevelse [OS] rate på 78 % [median oppfølging, 7 måneder; rekkevidde, 1–24 måneder]).
TOWER-studien (en fase 3, randomisert, åpen undersøkelse som undersøker effekten av BiTE-antistoffet Blinatumomab versus standardbehandling kjemoterapi hos voksne pasienter med residiverende/refraktær B-forløper ALL; clinicaltrials.gov identifikator NCT02013167) sammenlignet effektene av phoc-refraktær B-forløper ALL mot S-pretumomab-kjemikalier mot voksne blinaterapi med monoterapi hos voksne. -negativ, R/R BCP-ALL. Fordi folk levde lenger, ble studien stoppet tidlig. Bivirkninger i blinatumomab-gruppen var de samme som ble sett i tidligere studier, og blinatumomab hadde lavere eksponeringsjusterte AE-rater enn SOC.34 Blinatumomab virker også for personer med Ph-positiv, R/R BCP-ALL og for barn med Ph-negativ, R/R BCP-ALL.
30% to 50% of people with BCP-ALL in complete hematologic remission show persistent MRD. In the single-arm, phase 2 BLAST study (A Confirmatory Multicenter, Single-Arm Study to Assess the Efficacy, Safety, and Tolerability of the BiTE Antibody Blinatumomab in Adult Patients With MRD of B-Precursor Acute Lymphoblastic Leukaemia; clinicaltrials.gov identifier NCT01207388), blinatumomab was tested on patients with BCP-ALL in first or later complete After blinatumomab treatment, 78% of patients who were MRD positive became MRD negative. The 5-year OS study showed a median OS of 36.5 months, and more than half of those who had a complete MRD response after the first cycle of blinatumomab were still alive at 5 years, which suggests that the treatment might be able to cure some patients. AEs were seen that were linked to cytokinfrigivelsessyndrom (CRS).31 Other studies, like NCT03023878 and NCT03340766, are still looking at blinatumomab in first-line settings and in combination with other treatments.
CD19-targeted treatments have been linked to failure because of the loss of CD19 antigen after treatment. The failure rates for blinatumomab range from 8% to 35%, and for BIL T-celle terapier, they range from 39% to 65%.36-40 We don’t fully understand what causes therapy to fail, but one possibility is immunoediting, in which antigen loss is caused by a T-cell-dependent process called immunoselection, which lets tumour cells get away.41 Lineage switch and epitope loss under therapy pressure have also been suggested as ways for tumours to escape treatment. However, a recent study on epitope loss found that some CD19 isoforms that help CAR T-cells escape were already present at the time of diagnosis. This suggests that combining treatments might be helpful. Another thing that can cause immunotherapy to fail is called “inhibitory T-cell signalling.” In this case, the blocking programmed death ligand-1 (PD-L1) is interesting because it is more common in B-cell ALL cells from patients who don’t respond to blinatumomab and can make CD3 BiTE molecules less effective.43 By making a CD28/PD-L1 BiTE that triggers the CD28 co-stimulatory signal instead of the inhibitory signaling pathway that is usually seen when a T cell binds to a PD-L1-expressing cancer cell, this inhibition could be turned off.43 Dual-targeted CAR T cells are also being looked into as a way to make up for the loss of tumour antigens. This can be done by modifying each T cell with 2 CAR molecules and 2 different binding domains (dual-signaling CAR) or by putting 2 different binding domains on 1 CAR molecule at the same time (TanCAR).
Uønskede hendelser med BiTE og dets ledelse
I kliniske studier av blinatumomab er de vanligste bivirkningene feber, lavt antall hvite blodlegemer og lavt antall blodplater. Noen av de viktigste risikoene er CRS, nevrotoksisitet og narkotikafeil. Nevrotoksisitet kan også skje med CD19-spesifikke CAR T-cellebehandlinger, men det er kanskje ikke på grunn av CD19. Resultatene av en fase 1/1b-studie som fortsatt pågår om CD20/CD3-mål, viste at CNS AE av grad 3 eller høyere var sjeldne (3 % av alle grad 3 AE). Mesteparten av tiden er blinatumomabs reaksjon på CRS mild, men i sjeldne tilfeller kan den være alvorlig og til og med livstruende. Inflammatoriske reaksjoner kan reduseres med kortikosteroider. For å redusere sjansen for CRS er det best å gi en infusjon av prednison eller deksametason før første dose blinatumomab og å øke dosen sakte. Denne bruken av kortikosteroider før andre BiTE-molekyler har gitt grunn til å bruke deksametason som premedisinering ved bruk av andre BiTE-molekyler. Det er imidlertid ikke klart om denne effekten kan brukes på hele BiTE-plattformen, og andre måter å håndtere CRS på blir undersøkt. Interleukin 6 er et cytokin som forårsaker CRS og er høyt hos personer som har det. Tocilizumab, som blokkerer interleukin-6-reseptoren, har blitt brukt til å behandle CRS som er svært dårlig etter CAR T-cellebehandling.49 På sykehuset har tumornekrosefaktorhemmere også blitt brukt til å behandle CRS.


