
Carteyva, JW Therapeutics, lymphoma, refractory follicular lymphoma, RELIANCE study, relmacabtagene autoleucel
JW Therapeutics Announces NMPA Approval of Relmacabtagene Autoleucel Injection in Patients with Relapsed or Refractory Follicular Lymphoma
SHANGHAI, CHINA, October 10, 2022 - JW Therapeutics (HKEX: 2126), an independent and innovative biotechnology company focusing on developing, manufacturing, and commercializing cell immunotherapy products, announced that the Na..

BRL Biotech, CAR T-Cell, CARsgen Therapeutics, China CAR T Cell, IASO Biotherapeutics, Juventas Therapeutics, Relma-cel
How China is leading the development of CAR T-Cell therapy?
March 2023: CAR-T-cell therapy is a novel and effective cancer treatment modality that has revolutionized the treatment of cancer, particularly blood cancers. This therapy achieves a therapeutic effect or cures disease by repairi..

CAR19, CARGO, CD19, CD22, CRG-022, Gina Chapman, Third Rock Ventures
Cargo, a cancer drug startup, raises $200 million to combat CAR-T relapse
March 2023: Several types of lymphoma, as well as certain leukaemias and multiple myeloma, can now be effectively treated with CAR-T therapy. Recent studies have demonstrated the efficacy of CAR-T therapy at an earlier stage, res..

CAR - T Cell therapy, CAR T-Cell, Emily Littlejohn, Immunology, lupus renaissance
New CAR T-Cell therapy drug in lupus renaissance
Feb 2024: Several new drugs and promising therapies, such as chimeric antigen receptor T-cell therapy, have ushered in a "renaissance" for lupus, according to a speaker at the symposium Basic and Clinical Immunology for the Busy ..

Bruce Levine, CAR-T therapies, Carl June, Gilead, Kite, Tmunity Therapeutic, University of Pennsylvania
Kite completes acquisition of Tmunity
Press Release Feb 2023: - Kite, a Gilead Company (NASDAQ: GILD), today announced the completion of the previously announced transaction to acquire Tmunity Therapeutics (Tmunity), a clinical-stage, private biotech compan..
CD19-directed CAR T-cell therapy, CD22 CAR T, Large B-cell lymphoma, Matthew J. Frank, NCT04088890
High CR rates are overcome by CD22-directed CAR T-Cell therapy against CD19 relapse in LBCL
In February 2023, a phase 1 trial at a single institution found that it was safe and possible for people with heavily pretreated large B-cell lymphoma (LBCL) to use CD22-directed chimeric antigen receptor (CAR) T-cell therapy aft..

Abecma, idecabtagene vicleucel, KarMMa-3
CAR T-cell treatment provides significant remission rates in clinical trials
Feb 2023: In a Phase III study, a CAR T-cell therapy more than tripled progression-free survival compared with standard care for triple-class exposed multiple myeloma. Abecma (idecabtagene vicleucel) chimeric antigen receptor ..
ASTCT, CIBMTR, Large B-cell lymphoma, Matthew Frank, Miltenyi Biotec, Stanford University
Novel treatment target for lymphoma patients who relapse after CAR-T therapy
Feb 2023: The results of the trial demonstrated that a novel chimeric antigen receptor T-cell therapy elicited a response in adults with advanced large B-cell lymphoma who had relapsed following prior CAR-T. According to stati..
anti-BCMA, CAR - T Cell therapy, CT103A, FDA, IASO Biotherapeutics, Innovent Biologics, orphan drug designation
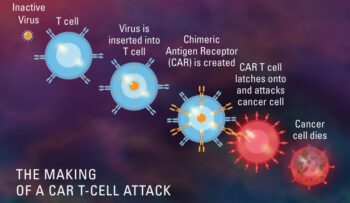
CT103A, CAR T-cell Therapy, has been designated as an orphan drug by the FDA
Feb 2023: The FDA has granted orphan drug status to CT103A, an experimental CAR T-cell therapy being developed by IASO Biotherapeutics and Innovent Biologics to treat relapsed or refractory multiple myeloma. Orphan drug design..
CAR - T Cell therapy, China, IASO Biotherapeutics, multiple myeloma
CAR T-cell treatment from IASO Biotherapeutics receives new FDA approval
Feb 2023: IASO Biotherapeutics' investigational CAR T-cell therapy for relapsed or refractory multiple myeloma (RRMM), CT103A, has received fast track and regenerative medicine advanced therapy designations from the U.S. Food and..