Latest news in cancer
Datopotamab deruxtecan-dlnk is approved by the USFDA for EGFR-mutated non-small cell lung cancer
On June 23, 2025, the Food and Drug Administration conferred accelerated approval to datopotamab deruxtecan-dlnk (Datroway, Daiichi Sankyo, Inc.) for adults...
Tafasitamab-cxix is approved by the USFDA for relapsed or refractory follicular lymphoma
On June 18, 2025, the US FDA approved tafasitamab‑cxix (Monjuvi), in combination with lenalidomide and rituximab, for adults with relapsed or refractory...
PiggyBac Transposon System: A Revolutionary Tool in Cancer Gene Therapy
The PiggyBac Transposon System is revolutionizing genetic engineering in cancer research, especially in CAR T-cell therapy. With its high integration...
Breakthrough Treatments for Advanced Breast Cancer in 2025
In 2025, advanced breast cancer treatment has entered a new era. Precision medicine and targeted therapies are enabling doctors to tailor care based on genetic...
Neoadjuvant and adjuvant pembrolizumab is approved by the USFDA for resectable locally advanced head and neck squamous cell carcinoma
On June 12, 2025, the FDA approved pembrolizumab for both neoadjuvant and adjuvant use in adults with resectable locally advanced head and neck squamous...
Mitomycin intravesical solution is approved by the USFDA for recurrent low-grade intermediate-risk non-muscle invasive bladder cancer
On June 12, 2025, the FDA approved mitomycin intravesical solution (brand name Zusduri, formerly UGN‑102) as the first non‑surgical, chemoablative...
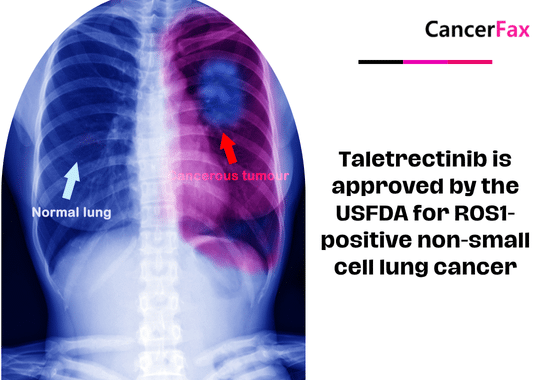
Taletrectinib is approved by the USFDA for ROS1-positive non-small cell lung cancer
On June 11, 2025, the FDA approved taletrectinib (brand name Ibtrozi) for adults with locally advanced or metastatic ROS1‑positive non‑small cell lung...
Darolutamide is approved by the USFDA for metastatic castration-sensitive prostate cancer
On June 3, 2025, the FDA approved darolutamide (brand name Nubeqa) for the treatment of adult patients with metastatic castration-sensitive prostate cancer...
Atezolizumab Plus Chemotherapy Improves Survival in Advanced-Stage Small-Cell Lung Cancer: Insights from the IMpower133 Study
The IMpower133 study highlights a breakthrough in treating extensive-stage small-cell lung cancer (ES-SCLC). Adding atezolizumab, a PD-L1 inhibitor, to...
Satri-cel CAR T-Cell Therapy: A New Era in Gastric Cancer Treatment
Satri-cel, a CLDN18.2-targeted CAR T-cell therapy, has shown promise in treating advanced gastric and gastroesophageal junction cancers. In a phase II trial,...
Shanghai Pulmonary Hospital’s Breakthrough Treatment Offers New Hope for Global Lung Cancer Patients
Shanghai Pulmonary Hospital has introduced a groundbreaking lung cancer treatment that is offering renewed hope to patients worldwide. With advanced...
Advancing Alström Syndrome Treatment in China: Gene Therapy and Clinical Innovations at NCMC, Shanghai
Alström Syndrome is a rare genetic disorder with no current cure, but emerging gene therapies in China offer new hope. At NCMC, Shanghai, cutting-edge...
Advancing Bardet-Biedl Syndrome Treatment in China: Gene Therapy and Clinical Innovations at NCMC, Shanghai
Certainly! Here's the **70-word excerpt** for the **previous blog post** on **Bardet-Biedl Syndrome**:
---
Bardet-Biedl Syndrome (BBS) is a rare genetic...
Advanced Innovative Treatment Clinic for Gliomas (CURE) in Tiantan Hospital, China
Beijing Tiantan Hospital stands at the forefront of glioma treatment in China, offering advanced therapies and participating in pioneering clinical trials....
Review and Prospect of Brain Glioma Treatment in China
China is advancing in brain glioma treatment through updated clinical guidelines and innovative therapies like immunotherapy and CAR-T cell therapy. Despite...
Shen Baiyong’s Pancreatic Cancer Innovation Studio in Shanghai, China
Dr. Shen Baiyong’s Pancreatic Cancer Innovation Studio at Ruijin Hospital, Shanghai, is pioneering advancements in early detection, precision treatment, and...
Safety and Tolerability of VGB-R04 in Patients With Hemophilia B
This Chinese trial is evaluating VGB-R04, a new gene therapy for Hemophilia B that uses a single AAV infusion to restore Factor IX activity. The study focuses...
A Study to Evaluate the Safety and Efficacy of ZS801 in Adult Hemophilia B Patients
A phase I/II gene therapy trial in China is testing ZS801, an AAV vector delivering the human Factor IX gene for adults with Hemophilia B. Conducted at the...
A Clinical Study Evaluating the Safety and Efficacy of SKG0201 Injection in Patients With Spinal Muscular Atrophy Type 1
A groundbreaking gene therapy trial in China is testing SKG0201, a single-dose rAAV-based treatment for Spinal Muscular Atrophy Type 1 (SMA 1). Conducted by...
GC101 Gene Therapy for Spinal Muscular Atrophy Type 1 – Phase I/II Clinical Trial
This Phase I/II trial tests GC101 gene therapy in infants with Type 1 Spinal Muscular Atrophy. Delivered intrathecally as a one-time treatment, GC101 aims to...
Evaluation of Safety and Efficacy of Gene Therapy Drug in the Treatment of Spinal Muscular Atrophy (SMA) Type 2 Patients
This Phase I/II trial investigates GC101, a gene therapy using AAV9 vectors to treat children with Spinal Muscular Atrophy Type 2. Administered as a one-time...
Evaluation of Safety and Efficacy of Gene Therapy Drug in the Treatment of Spinal Muscular Atrophy (SMA) Type 3 Patients
This clinical trial investigates the safety and efficacy of GC101 gene therapy in treating Type 3 Spinal Muscular Atrophy (SMA). The study involves a single...
Safety and Efficacy Evaluation of GC101 Gene Therapy Via Intrathecal (IT) Injection in the Treatment of Patients With Type 2 Spinal Muscular Atrophy (SMA) – Phase III
This Phase III clinical trial evaluates GC101 gene therapy for children aged 2–12 with Type 2 Spinal Muscular Atrophy (SMA). Sponsored by GeneCradle Inc.,...
Retifanlimab-dlwr with carboplatin and paclitaxel is approved by the USFDA and as a single agent for squamous cell carcinoma of the anal canal
On May 15, 2025, the FDA approved Zynyz (retifanlimab-dlwr) in combination with carboplatin and paclitaxel, and as a single agent, for treating adults with...
Accelerated approval is granted by the USFDA to telisotuzumab vedotin-tllv for NSCLC with high c-Met protein overexpression
On May 14, 2025, the FDA granted accelerated approval to Emrelis (telisotuzumab vedotin) for treating adults with high c-Met protein overexpressing non-small...
Belzutifan is approved by the USFDA for pheochromocytoma or paraganglioma
On May 14, 2025, the FDA approved belzutifan (Welireg) for treating adults and children aged 12 and older with locally advanced, unresectable, or metastatic...
Accelerated approval is granted by the USFDA to the combination of avutometinib and defactinib for KRAS-mutated recurrent low-grade serous ovarian cancer
The FDA has granted accelerated approval to the combination therapy of avutometinib and defactinib for treating KRAS-mutated recurrent low-grade serous ovarian...
Chimerix’s Dordaviprone Receives FDA Priority Review for Recurrent H3 K27M-Mutant Diffuse Glioma
Chimerix’s drug Dordaviprone has received FDA priority review for treating recurrent H3 K27M-mutant diffuse glioma. With a PDUFA date of August 18, 2025, it...
Penpulimab-kcqx is approved by the USFDA for non-keratinizing nasopharyngeal carcinoma
Penpulimab-kcqx, developed by Akeso Biopharma, has received FDA approval for treating recurrent or metastatic non-keratinizing nasopharyngeal carcinoma. This...
Dr. Zhang Wei: Leading Innovation in Epigenetic Therapy for Diffuse Midline Glioma at Tsinghua University
Dr. Zhang Wei of Tsinghua University leads pioneering research in epigenetic-based immunotherapy for Diffuse Midline Glioma (DMG). His work integrates advanced...
Epigenetic Agent-Based Immunotherapy Shows Promise in Diffuse Midline Glioma
A groundbreaking case study reveals that epigenetic agent-based immunotherapy significantly regressed tumors in a patient with diffuse midline glioma (DMG),...
Breakthrough in Cancer Treatment: FDA Approves Drug from Small Enterprise in Tianjin, China
Introduction In a stunning surprise that has stunned the international biotech industry, a tiny and micro business in Tianjin, China, has come up with an...
ONC201 Capsules: A New Hope for Brain Glioma Patients in China
Gliomas Introduction Brain gliomas, especially aggressive types such as glioblastoma multiforme (GBM), have long been extremely challenging to treat because of...
Medical Tourism in China: Best Hospitals, Costs & Treatments (2025 Guide)
China has become a leading destination for medical tourism, providing high-end treatment at 30-70% lower prices compared to Western nations. Ranging from...
4th Generation CAR T Cell Therapy with IL-15: A new era in cancer treatment
4th generation CAR T-cell therapy with IL-15 represents a groundbreaking advancement in cancer immunotherapy. By incorporating IL-15, these engineered T cells...
Nivolumab with ipilimumab is approved by the USFDA for unresectable or metastatic hepatocellular carcinoma
On April 11, 2025, the Food and Drug Administration sanctioned nivolumab (Opdivo, Bristol Myers Squibb Company) in conjunction with ipilimumab (Yervoy, Bristol...
Need help? Our team is ready to assist you.
We wish a speedy recovery of your dear and near one.