anti-BCMA, CAR - T Cell therapy, CT103A, FDA, IASO Biotherapeutics, Innovent Biologics, orphan drug designation
CT103A, CAR T-cell Therapy, has been designated as an orphan drug by the FDA
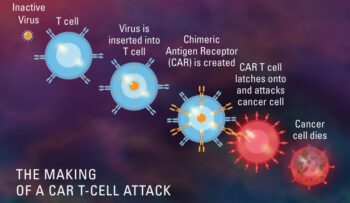
Feb 2023: The FDA has granted orphan drug status to CT103A, an experimental CAR T-cell therapy being developed by IASO Biotherapeutics and Innovent Biologics to treat relapsed or refractory multiple myeloma. Orphan drug design..