Feb 2023: The FDA has granted orphan drug status to CT103A, an experimental CAR T-cell therapy being developed by IASO Biotherapeutics and Innovent Biologics to treat relapsed or refractory multiple myeloma.
Orphan drug designation is given to therapies that have the potential to significantly improve care for rare diseases, which are defined as conditions that affect 200,000 or fewer people in the United States. It provides incentives to the therapy’s developers; for example, orphan drugs are eligible for seven years of market exclusivity if approved by the FDA.
This status “is of great significance to patients with multiple myeloma and represents the FDA’s recognition of CT103A and the clinical data provided by IASO Bio,” Wen Wang, MD, PhD, CEO and chief medical officer of IASO, said in a press release.
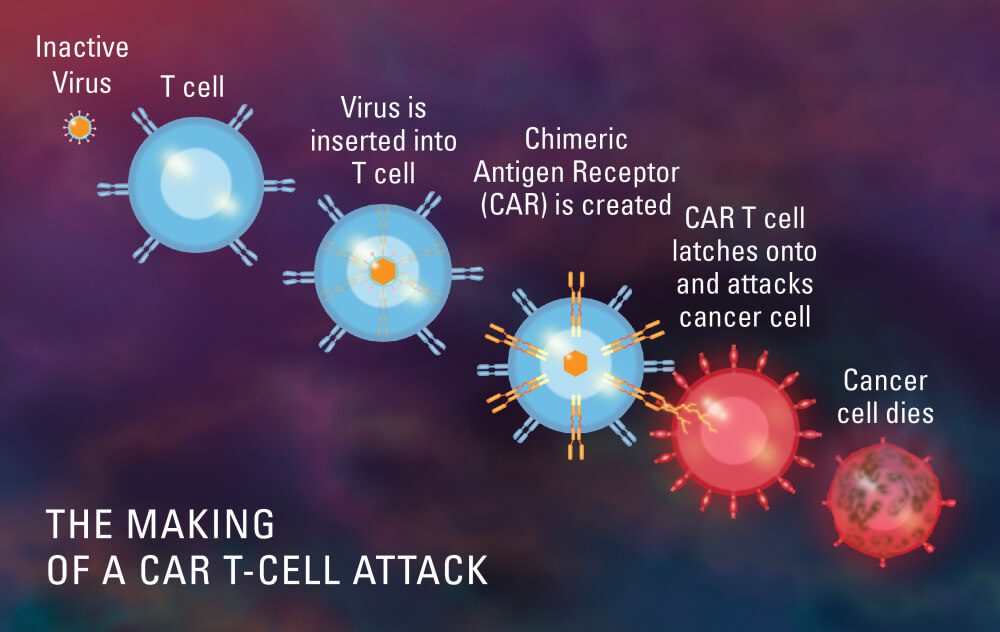
T-cells, which are immune cells, have the ability to kill cancerous cells, but tumour cells frequently find ways to avoid immune-mediated destruction.
CT103A (also known as IBI326) is a CAR T-cell therapy. Simply put, this type of therapy involves imbuing T-cells with a specially designed protein receptor known as a chimeric antigen receptor (CAR), which allows them to more effectively identify and target cancer cells.
CT103A specifically equips T-cells to target a protein known as B-cell maturation antigen (BCMA), which is commonly expressed on the surface of myeloma cells.
According to IASO, BCMA-targeting CAR-T cells can induce remission in patients with multiple myeloma. However, they are known to be immunogenic (the development of immune responses against the treatment that prevents repeat dosing) and to lose effectiveness over time, making relapses likely. CT103A’s specific receptor for T-cells is intended to address these issues.
In an open-label Phase 1 clinical trial (ChiCTR1800018137), 18 relapsed or refractory multiple myeloma patients with a median of four prior therapy lines were given increasing doses of CT103A. All had responded to the treatment after a median of 394 days (a little more than a year), with 72.2% achieving complete responses or better.
All patients with available data were also negative for minimal residual disease, which refers to the small number of cancer cells that can remain after treatment and cause disease relapse.
The trial’s researchers reported that “short persistence of CAR T cells in vivo [in a living body] may be one of the most important reasons for BCMA-positive relapse.”
“A second-generation anti-BCMA CAR [therapy] with a fully human component was used in the CT103A trial,” they added.
“Our effort to develop a novel anti-BCMA CAR with improved efficacy and persistence” has earned the drug orphan status. It emphasises the importance of bringing this therapeutic option to patients with multiple myeloma and strongly motivates us to accelerate IBI326 clinical development,” said Hui Zhou, PhD, senior vice president of Innovent.
“We hope to launch CT103A as soon as possible in both China and the United States,” Wang added.