Bristol-Myers Squibb, CHECKMATE-76K, FDA, melanoma, Opdivo
Nivolumab is approved by the FDA for adjuvant treatment of Stage IIB/C melanoma
Nov 2023: Nivolumab (Opdivo, Bristol-Myers Squibb Company) was granted approval by the Food and Drug Administration as an adjuvant therapy for Stage IIB/C melanoma in patients 12 years of age and older who had undergone complete..
anti-BCMA, CAR - T Cell therapy, CT103A, FDA, IASO Biotherapeutics, Innovent Biologics, orphan drug designation
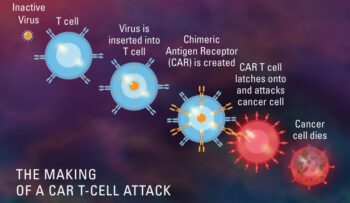
CT103A, CAR T-cell Therapy, has been designated as an orphan drug by the FDA
Feb 2023: The FDA has granted orphan drug status to CT103A, an experimental CAR T-cell therapy being developed by IASO Biotherapeutics and Innovent Biologics to treat relapsed or refractory multiple myeloma. Orphan drug design..

Eli Lilly and Company, FDA, Food and Drug Administration, Jaypirca, Mantle cell lymphoma, MCL, pirtobrutinib
Accelerated approval is granted by FDA to pirtobrutinib for relapsed or refractory mantle cell lymphoma
Feb 2023: Accelerated approval is granted by FDA to pirtobrutinib (Jaypirca, Eli Lilly and Company) for relapsed or refractory mantle cell lymphoma. In BRUIN (NCT03740529), an open-label, multicenter, single-arm trial of pirtobr..
FDA, Food and Drug Administration, KEYTRUDA, Merck, Pembrolizumab, platinum-based chemotherapy
Pembrolizumab is approved by FDA as adjuvant treatment for non-small cell lung cancer
Feb 2023: For stage IB (T2a 4 cm), stage II, or stage IIIA non-small cell lung cancer, the Food and Drug Administration (FDA) approved pembrolizumab (Keytruda, Merck) as adjuvant therapy after resection and platinum-based chemoth..
FDA, Food and Drug Administration, NCT03438396, tisotumab vedotin-tftv, Tivdak
Tisotumab forvedotin-tftv is approved for recurrent or metastatic cervical cancer
October 2021: The FDA has given tisotumab vedotin-tftv (Tivdak, Seagen Inc.), a tissue factor-directed antibody and microtubule inhibitor combination, rapid approval for adult patients with recurrent or metastatic cervical cancer ..
CD-38, FDA, HORIZON, immunomodulatory agent, melphalan flufenamide, multiple myeloma, NCT02963493, Oncopeptides AB, Pepaxto
Melphalan flufenamide receives approval from the FDA for relapsed or refractory multiple myeloma
August 2021: Melphalan flufenamide (Pepaxto, Oncopeptides AB) in combination with dexamethasone has been granted accelerated approval by the Food and Drug Administration for adult patients with relapsed or refractory multiple myel..
cemiplimab-rwlc, EGFR, FDA, Food and Drug Administration, NCT03088540, NSCLC, PD-L1 expression
Cemiplimab-rwlc has been approved by the FDA for non-small cell lung cancer with high PD-L1 expression
August 2021: The FDA approved cemiplimab-rwlc (Libtayo, Regeneron Pharmaceuticals, Inc.) for the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC) (locally advanced who are not candidates for surgic..
FDA, KRAS G12C, Lumakras, NSCLC, sotorasib
The FDA has approved the first targeted therapy for a lung cancer mutation that was previously thought to be drug-resistant
August 20, 2021: Recently in May, 2021 Lumakras (sotorasib) was approved by the US Food and Drug Administration as the first treatment for adult patients with non-small cell lung cancer who have undergone at least one prior system..
FDA, Lonsurf, TAGS (NCT02500043), tipiracil tablets, Trifluridine
Lonsurf is approved by FDA for recurrent, metastatic gastric and gastroesophageal junction adenocarcinoma
Trifluridine/tipiracil tablets (LONSURF, Taiho Pharmaceutical Co., Ltd.) were approved by the Food and Drug Administration on February 22, 2019 for adult patients with metastatic gastric or gastroesophageal junction (GEJ) adenocar..
Ablynx, ADAMTS13, CABLIVI, caplacizumab-yhdp, FDA, immunosuppressive therapy, USFDA
Caplacizumab-yhdp approved by FDA
On February 6, 2019, in conjunction with plasma exchange and immunosuppressive treatment, the Food and Drug Administration approved caplacizumab-yhdp (CABLIVI, Ablynx NV) for adult patients with acquired thrombotic thrombocytopeni..