CAR T-Cell therapy for non-small cell lung cancer
A revolutionary approach in the treatment of lung cancer.
Want to enroll in this breakthrough cancer treatment?
March 2024 : CAR T-cell therapy is a potential cancer treatment strategy, notably in hematological malignancies. However, its effectiveness in solid tumors, such as lung cancer, is limited due to the tumor microenvironment’s immunosuppressive nature. Researchers are developing next-generation CAR T cells to boost their infiltration, survival, and persistence inside malignancies. Clinical trials are underway to assess the safety and efficacy of CAR T-cell treatments in lung cancer, with some yielding promising results. Antigen escape, immunological barriers, and on-target off-tumor damage are among the challenges in CAR T-cell treatment for lung cancer. Engineering CAR constructs, altering the tumor microenvironment, and employing off-the-shelf CAR T cells are some strategies for addressing these problems.
One of the leading hospitals in China has successfully conducted trials of CAR T-Cell therapy in non-small cell lung cancer patients. CAR T-Cell on all these cancers is applicable for patients after some lines of treatment such as surgery, chemotherapy and radiotherapy but relapsed.
Of all malignancies, lung cancer has the greatest incidence and fatality rates worldwide. A growing variety of immunotherapeutic medicines, particularly those that target monoclonal antibodies, have been employed in the clinical treatment of malignancy in the current immunotherapy period, although it still has numerous drawbacks. In addition to being utilised successfully against haematological cancers, chimeric antigen receptor-modified T (CAR-T) cells have also created new opportunities for the immunotherapy of solid tumours, such as lung cancer. The lack of appropriate tumor-specific antigens, an immunosuppressive tumour microenvironment, a low level of CAR-T cell penetration into tumour tissues, together with off-target effects, etc. make it difficult to target lung cancer-specific antigens with modified CAR-T cells. Meanwhile, due to numerous difficulties such as tumor lysis syndrome, neurotoxicity syndrome, and cytokine release syndrome, the clinical usage of CAR-T cells is still restricted. With the goal of offering fresh perspectives and methods for pre-clinical studies and clinical trials of CAR-T cell therapy for lung cancer, we outline the fundamental structure and generation characteristics of CAR-T cells in this review, summarise the typical tumor-associated antigens, and highlight the current challenges.
The structure of CARS
Since its inception, the use of CARs in T-cell therapy has gone through four iterative generations, all of which are based on the CAR’s intracellular signal domains. The first generation of CARs had weak activity and a brief in vivo survival time because they only contained the antigen recognition signal. The signal transduction area of the second and third generation CARs, respectively, contained one and two costimulatory molecules. These changes were made in order to increase T cell survival, cytotoxicity, and proliferation. The co-stimulatory molecules in the CARs were improved, which improved CAR-T cell performance. 4-1BB or CD28 are the two second-generation co-stimulatory domains that are most frequently utilised. Additionally, cytotoxicity, cytokine production, and T cell activation have all been demonstrated to be improved by DNAX-activating protein 10 (DAP10). Based on non-small cell lung cancer (NSCLC) cell lines, delayed initial lung cancer growth and increased anti-tumor activity were demonstrated in in vivo animal models of human lung cancer xenotransplantation. Pro-inflammatory cytokines and co-stimulating ligands were added in the fourth generation CAR-T design to help the T-cells infiltrate and get beyond the hostile TME’s suppressive properties.
Amplification and anti-tumor efficacy of CAR-T cells have been demonstrated to be enhanced by improving the extracellular module structure in addition to intracellular signal transduction modules. According to Qin et al., the single-chain variable fragment (scFv), which binds to and encourages the expansion, migration, and invasion of cluster of differentiation 4 (CD4)+ CAR-T cells, was made more flexible by the addition of a hinge structure. Though second-generation CAR-T cells continue to be the standard method for therapeutic application, the structural architecture of the CARs is constantly being improved and is crucial to CAR-effectiveness. T’s
CAR T-Cell therapy in lung cancer and target antigen
When the target-antigen is exclusively expressed on cancer cells or is overexpressed on all or the majority of lung cancer cells in comparison to normal cells, this is the best target for CAR-T cell treatment. Although a large number of tumor-associated antigens (TAA) have been found in non-small cell lung cancers (NSCLCs), only a few of these antigens have been specifically targeted by CAR-T cells (8). Additionally, some of these target-antigens are also weakly expressed in healthy tissues, giving some CAR-T cells the ability to assault healthy cells.
Epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), mesothelin (MSLN), prostate stem cell antigen (PSCA), mucin 1 (MUC1), carcinoembryonic antigen (CEA), tyrosine kinase-like orphan receptor (ROR1), programmed death ligand 1 (PD-L1), and CD80/CD86 are among the targets currently being studied for CAR.
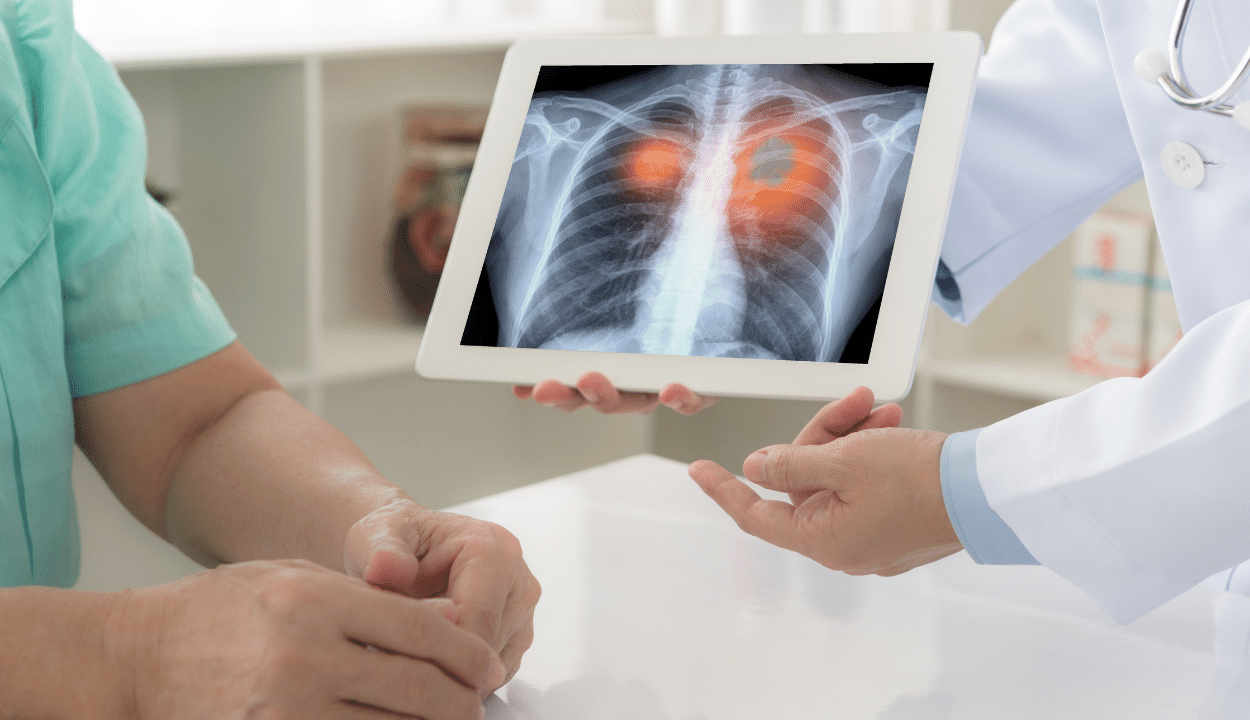
Lung cancer patient undergoing CAR T-Cell therapy
November of 2009, the patient found a left lung mass and underwent radical left lung cancer radical surgery. Pathology: lung adenocarcinoma;
From Jan 2013to Jan 2017, three brain metastases occurred, and surgery and radiation therapy were given successively with poor control;
From March 2017 to September 2017, for brain metastases, mesoCAR-αPD1 cells expressing PD-1 antibody were given for 6 courses of treatment. After treatment, PR was evaluated and tumors shrank significantly with only a small amount of residue.
CAR T-Cell therapy in China
CAR-T Cell therapy in China is growing at a very rapid pace. Results of CAR T-cell therapy in China and overall cure rate is among the best in the world at present. There are more than 300 clinical trials that are taking place in China for CAR T Cell therapy. China is among the first countries to offer CAR T Cell therapy after USA & UK. In terms of the number of CAR-T clinical trials, China is second to the USA, registering approximately 33% of trials worldwide. The number of CAR T-cell therapies in clinical development has rocketed in recent years. Currently, in China, there are over 300 ongoing clinical trials in hematologic malignancies as well as solid tumors.
China’s extensive explorations and breakthroughs in the search of novel target antigens, optimization of CAR structure, cocktail CAR-T therapy, combination therapy, and extension of CAR-T cell applications, imply that we are currently on the verge of a revolution in CAR-T therapy. US FDA has approved CAR T Cell therapy for relapsed B Acute lymphoblastic leukemia, lymphoma & multiple myeloma. China has recently approved CAR T-Cell therapy for some solid cancers. Patients from across the world are likely to benefit from this development.
You may like to read: CAR T-Cell therapy in China
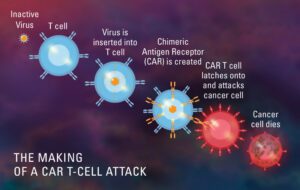
What is CAR T-Cell therapy (Chimeric antigen receptors)?
CAR T-Cell therapy is a form of immunotherapy that uses specially modified T-cells which are part of our immune system to fight cancer. A sample of patients T cells are collected from the blood, then it is modified to produce special structures called chimeric antigen receptors (CAR) on their surface. When these modified CAR cells are re infused in the patient, these new cells attack the specific antigen and kills the tumor cells.
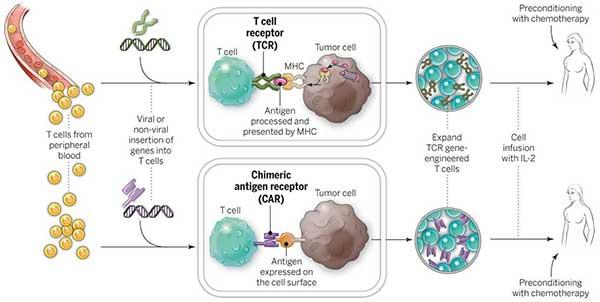
How does CAR T-Cell therapy work?
CAR T-cell therapy takes help from body’s own immune system to attack and kill cancer cells. This is done by removing some specified cells from the blood of the patient, modifying them in the lab and re-injecting them into the patient. CAR T-cell therapy has produced very encouraging results in Non-Hodgkin lymphoma and thus approved by FDA.
Who are the right candidates for CAR T-Cell therapy ?
At present FDA has approved CAR T-Cell therapy for some forms of aggressive and refractory Non-Hodgkin lymphoma and relapsed and refractory acute lymphoblastic leukemia. Patient need to send full medical reports to ascertain the use of CAR T-Cell therapy for his treatment.
Inclusion criteria for CAR T-cell therapy :
1. Patients with CD19+ B-cell Lymphoma(At least 2 prior combination chemotherapy regimens)
2. To be aged 3 to 75 years
3. ECOG score ≤2
4. Women of childbearing potential must have a urine pregnancy test taken and proven negative prior to the treatment. All patients agree to use reliable methods of contraception during the trial period and until follow-up for the last time.
Exclusion criteria for CAR T-cell therapy :
1. Intracranial hypertension or unconsciousness
2. Respiratory failure
3. Disseminated intravascular coagulation
4. Hematosepsis or Uncontrolled active infection
5. Uncontrolled diabetes
Advantages of CAR T-Cell therapy
- >5000 CAR T cases done by highly skilled doctor’s.
- Hospitals in China has developed more CAR T Cell types including CD19 & CD 22 then any other country in the world.
- China is conducting more than 300 clinical trials on CAR T Cell therapy. More than any other country on the planet.
- Clinical effect of CAR T Cell is similar to that in USA or any other country and sometimes better.
Treatment process for CAR T-Cell therapy
- Complete evaluation of the patient
- T-cell collection from the body
- T-cells are then engineered in the lab
- Genetically engineered T-Cells are then multiplied by using growing them in the laboratory. These cells are frozen and then sent to the treating centres.
- Prior to infusing, patient may be given chemotherapy for their cancer. This helps the therapy work in a better way.
- Soon after chemotherapy CAR T-Cells are infused by a process which is similar to blood infusion.
- There is a 2-3 month of recovery period for the patient.
Time frame for CAR T-Cell therapy
1. Examination & test: one week
2. Pre-treatment & T-Cell Collection: one week
3. T-Cell preparation & return: two-three weeks
4. 1st Effectiveness analysis: three weeks
5. 2nd Effectiveness analysis: three weeks.
Side effects of CAR T-Cell therapy
The common side effects of CAR T-cell therapy include:
- Cytokine release syndrome
In some cases, patients may develop flu-like symptoms such as fever, chills, headache, nausea, vomiting, loose stools, and muscle or joint pains. It may also cause low blood pressure, difficulty in breathing, and a fast heart rate. These side effects are due to the release of cytokines by the immune cells during CAR T-cell therapy. These symptoms are usually mild, but can be serious and life threatening in some patients. - Neurological events
Neurological events can occur and can be serious in some patients. Such events include encephalopathy (brain injury and malfunction), confusion, difficulty speaking, agitation, seizures, drowsiness, altered state of consciousness and loss of balance. - Neutropaenia and Anaemia
Some patients may develop neutropenia or low white cell count. Similarly, anaemia or low red blood cell count may also occur due to this therapy.
.
Fortunately, most of these side effects usually resolve on their own or can be managed with the use of medications.
How effective is CAR T-Cell therapy ?
CAR T-cell therapy for the treatment of lymphoma and other blood cancers has shown promising outcomes. Since CAR T-cell treatment, many patients who had previously relapsed blood tumours had promising results and no evidence of cancer. It has also aided in the rehabilitation of patients who have previously failed to respond to most traditional cancer therapies.
However, longer-term studies for a larger patient population are needed to validate the efficacy of this treatment. Large-scale experiments would also aid in determining the likelihood of side effects and the right ways to deal with them.
How much does CAR T-Cell therapy cost ?
China is a world leader of CAR-T cell therapy & BMT. Till now there are more than 300 CAR-T cell clinical trials in progress. China’s CAR-T treatment is the most budget one around the world. Because the CAR-T cell preparation is free now ! The patients only have to pay for the treatment & services. Total cost of treatment will be around $60,000 -$80,000.
Also read this : CAR T Cell therapy in India
How can I take CAR T-Cell therapy in China?
Patient can call +91 96 1588 1588 or email to cancerfax@gmail.com with patient details and medical reports and we shall arrange for second opinion, treatment plan and estimate of expenses.