CAR T-Cell Therapy
Modern immunotherapy methods, like CAR T-cell therapy, have completely changed how cancer is treated. It entails genetically altering a patient’s own T cells so that they express CARs, or chimeric antigen receptors, which are able to recognize only cancer cells.
These cells are reinserted in the patient, and these altered CAR T cells can efficiently target and eliminate cancer cells. With high response rates and long-lasting remissions, CAR T-cell therapy has demonstrated extraordinary efficacy in treating specific forms of blood malignancies, such as leukaemia, lymphoma, and multiple myeloma.
What is CAR T-Cell therapy?
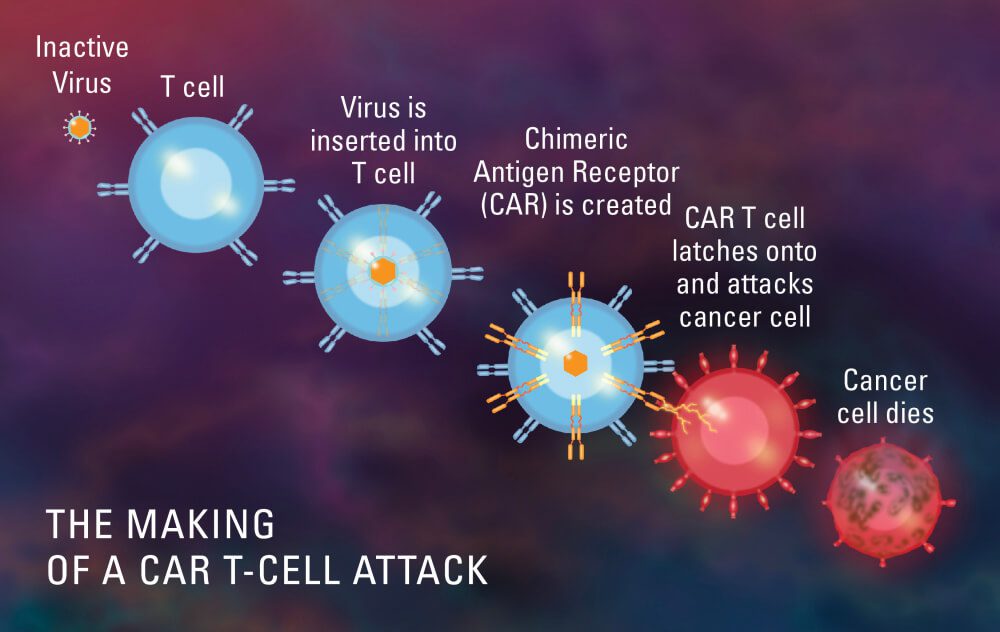
Chimeric antigen receptor T-cell therapy, often known as CAR T-cell therapy, is a ground-breaking immunotherapy that has completely changed the way that cancer is treated. It gives patients with certain cancers hope that was previously seen as incurable or with few therapeutic alternatives.
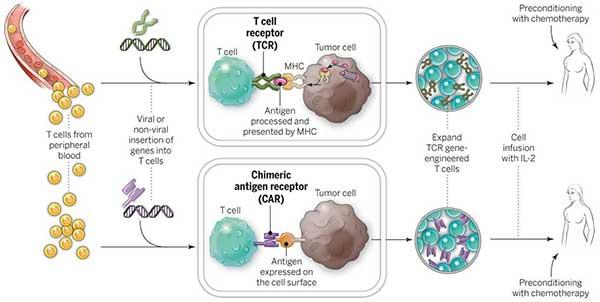
The treatment entails using a patient’s own immune cells, more specifically, T cells, and lab-modifying them to improve their capacity to detect and destroy cancer cells. To do this, the T cells are given a chimeric antigen receptor (CAR), which gives them the ability to target particular proteins, or antigens, on the surface of cancer cells.
T cells from the patient are first removed, and they are then genetically modified to express the CAR. In the laboratory, these altered cells are multiplied to produce a sizable population of CAR T cells, which are then put back into the patient’s bloodstream.
As soon as they are inside the body, CAR T cells find cancer cells that express the desired antigen, attach to them, and trigger a potent immune response. The CAR T cells that have been activated proliferate and conduct a focused attack on the cancer cells, killing them.
How does CAR T-Cell therapy work?
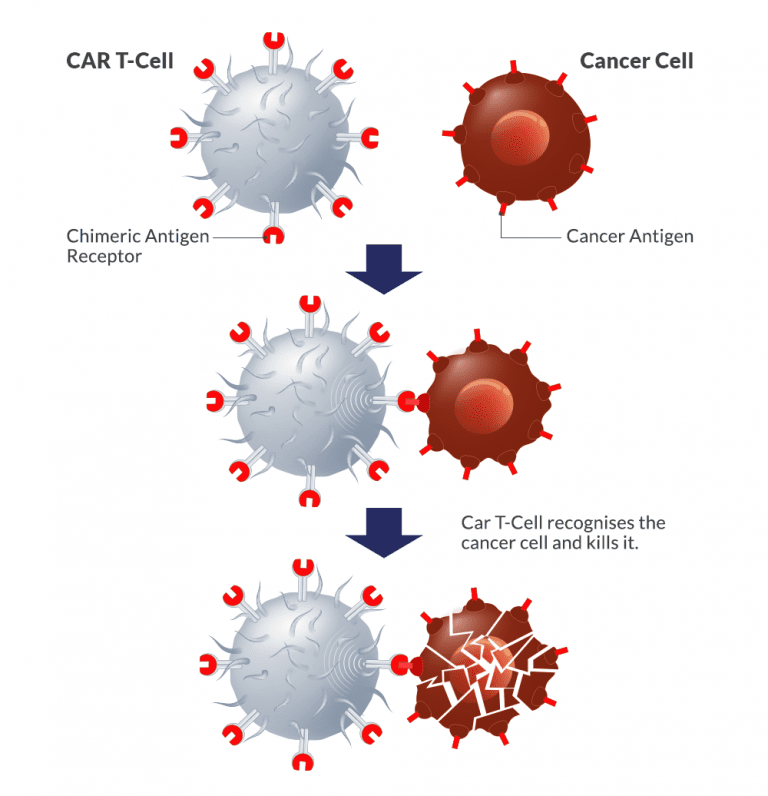
When used to treat some blood malignancies like acute lymphoblastic leukaemia (ALL) and specific forms of lymphoma, CAR T-cell therapy has shown exceptional results. It has produced notable response rates and, in some patients, even long-lasting remissions.
CAR T-cell therapy, however, is a sophisticated and unique therapeutic method that might have risks and adverse effects. Cytokine release syndrome (CRS), a widespread immunological reaction that can result in flu-like symptoms and, in extreme situations, organ failure, may be experienced by certain people. There have also been reports of neurological adverse effects; however, they are frequently curable.
Despite these difficulties, CAR T-cell therapy is a significant advancement in the fight against cancer and shows great potential for the future. Current studies are focused on enhancing its efficacy and safety profile as well as extending its use to different cancer types. CAR T-cell therapy has the ability to change the face of cancer treatment and give patients everywhere new hope with further advancements.
Procedure
The CAR-T therapy procedure, which takes a few weeks, involves multiple steps:
T cells are extracted from your blood using a tube that is placed into an arm vein. This takes a couple of hours.
T cells are transported to a facility, where they undergo genetic modification to become CAR-T cells. Two to three weeks pass through this.
CAR-T cells are reintroduced into your bloodstream through a drip. This requires several hours.
CAR-T cells target and eliminate cancer cells throughout the body. After receiving CAR-T therapy, you will be closely watched.
What type of cancer cells can be treated with CAR-T Cell Therapy?
Only patients with adult B-cell non-lymphoma Hodgkin’s or pediatric acute lymphoblastic leukemia who have already tried two unsuccessful conventional therapies can currently use CAR T-cell therapy products that have received FDA approval. However, CAR T-cell therapy is now being tested in clinical studies as a first- or second-line treatment for adult lymphoma and pediatric acute lymphoblastic leukemia. Recently, some of the studies have shown remarkable successes in cases of solid tumors too like glioblastoma, gliomas, liver cancer, lung cancer, GI cancer, pancreatic cancer and oral cancer.
To conclude
This represents a significant advancement in the management of leukemia and B-cell lymphoma. Additionally, it gives hope to those whose lives had previously been predicted to last only six months. Now that we have identified mechanisms of resistance and created more techniques to combat them, the future appears to be much more promising.
Get in touch with our highly experienced healthcare providers here at CancerFax for a free consultation to work out a suitable care plan for your healthcare needs. Please send your medical reports to info@cancerfax.com or WhatsApp to +1 213 789 56 55.

You may like to read: CAR T-Cell therapy in India
What are the advantages of CAR-T Cell Therapy?
The main benefit is that CAR T-cell therapy only requires a single infusion and often only requires two weeks of inpatient care. Patients with non-Hodgkin lymphoma and pediatric leukemia who have just been diagnosed, on the other hand, typically need chemotherapy for at least six months or more.
The advantages of CAR T-cell therapy, which is actually a living medication, can persist for many years. If and when a relapse occurs, the cells will still be able to identify and target cancer cells because they can survive in the body for an extended period of time.
Although the information is still developing, 42% of adult lymphoma patients who underwent CD19 CAR T-cell treatment were still in remission after 15 months. And after six months, two-thirds of patients with pediatric acute lymphoblastic leukemia were still in remission. Unfortunately, these patients had exceedingly aggressive tumors that weren’t successfully treated using traditional standards of care.
What type of patients would be good recipients of the CAR-T Cell Therapy?
Patients between the age of 3 Years to 70 Years have been tried with CAR T-Cell therapy for different type of blood cancers and has been found to be very effective. Many centers have claimed success rates of more than 80%. The optimum candidate for CAR T-cell therapy at this time is a juvenile with acute lymphoblastic leukemia or an adult with severe B-cell lymphoma who has already had two lines of ineffective therapy.
Before the end of 2017, there was no accepted standard of care for patients who had already gone through two lines of therapy without experiencing remission. The only FDA-approved treatment that has so far proven to be significantly beneficial for these patients is CAR T-cell therapy.
How effective is CAR-T Cell therapy?
CAR T-cell therapy has been very effective in treating some types of blood cancer, like acute lymphoblastic leukaemia (ALL) and non-Hodgkin lymphoma. In clinical trials, the response rates have been very good, and a lot of patients have gone into full remission. In some cases, people who had tried every other medicine had long-lasting remissions or even possible cures.
One of the best things about CAR T-cell treatment is that it targets the right cells. The CAR receptors that have been added to the T cells can find specific marks on cancer cells. This makes it possible to give targeted treatment. This targeted method hurts healthy cells as little as possible and lowers the risk of side effects that come with traditional treatments like chemotherapy.
But it’s important to keep in mind that CAR T-cell therapy is still a new area that is still changing. Researchers and doctors are working hard to solve problems like the high cost, the possibility of serious side effects, and the fact that it only works for some types of cancer.
In the end, CAR T-cell therapy has shown to be a very successful way to treat some types of blood cancer. Even though it is a promising and powerful method, more study and clinical trials are needed to improve it and find new ways to use it. CAR T-cell therapy could change how cancer is treated and make things better for people all over the world if it keeps getting better.

You may like to read: CAR T-Cell therapy in Israel
Inclusion & exclusion criteria
Inclusion criteria for CAR T-cell therapy:
1. Patients with CD19+ B-cell Lymphoma(At least 2 prior combination chemotherapy regimens)
2. To be aged 3 to 75 years
3. ECOG score ≤2
4. Women of childbearing potential must have a urine pregnancy test taken and proven negative prior to the treatment. All patients agree to use reliable methods of contraception during the trial period and until follow-up for the last time.
Exclusion criteria for CAR T-cell therapy:
1. Intracranial hypertension or unconsciousness
2. Respiratory failure
3. Disseminated intravascular coagulation
4. Hematosepsis or Uncontrolled active infection
5. Uncontrolled diabetes.
CAR T-Cell therapies approved by USFDA
B-cell precursor acute lymphoblastic leukemia, relapsed or refractory diffuse large B-cell lymphoma
Complete response rate (CR): >90%
Target: CD19
Price: $475,000
Approval time: August 30, 2017
Relapsed or refractory diffuse large B-cell lymphoma, relapsed or refractory follicular cell lymphoma
Non-Hodgkin’s lymphoma Complete response rate (CR): 51%
Target: CD19
Price: $373,000
Approval time: 2017 October 18
Relapsed or refractory diffuse large B-cell lymphoma
Mantle cell lymphoma Complete response rate (CR): 67%
Target: CD19
Price: $373,000
Approved time: October 18, 2017
Relapsed or refractory diffuse large B-cell lymphoma
Complete response rate (CR): 54%
Target: CD19
Price: $410,300
Approved time: October 18, 2017
Relapsed or Refractory Multiple Myeloma
Complete response rate: 28%
Target: CD19
Price: $419,500
Approved: October 18, 2017
What are the side effects of CAR-T Cell therapy?
Below mentioned are some of the side-effects of CAR T-Cell therapy.
- Cytokine release syndrome (CRS): The most prevalent and possibly significant side effect of CAR T-cell treatment is cytokine release syndrome (CRS). The flu-like symptoms, including fever, exhaustion, headaches, and muscle pain, are brought on by the modified T cells’ production of cytokines. In extreme circumstances, CRS may result in a high temperature, hypotension, organ failure, and even potentially fatal consequences.
- Neurological Toxicity: Some patients may develop neurological side effects, which can range in severity from less serious signs like mild confusion and disorientation to more serious ones like seizures, delirium, and encephalopathy. After CAR T-cell infusion, neurological toxicity frequently happens during the first week.
- Cytopenias: CAR T-cell treatment can result in low blood cell counts, such as anaemia (low red blood cell count), neutropenia (low white blood cell count), and thrombocytopenia (low platelet count). Infections, bleeding, and exhaustion are among risks that can be exacerbated by these cytopenias.
- Infections: The CAR T-cell therapy’s suppression of healthy immune cells increases the risk of bacterial, viral, and fungal infections. In order to prevent infections, patients may need to be closely watched and given preventive medication.
- Tumour Lysis Syndrome (TLS): After CAR T-cell therapy, it’s possible in some circumstances for substantial amounts of cell contents to be released into the bloodstream due to the rapid killing of tumour cells. This may result in metabolic abnormalities, such as excessive potassium, uric acid, and phosphate levels, which may damage the kidneys and cause other problems.
- Hypogammaglobulinemia: CAR T-cell treatment has the potential to decrease antibody synthesis, which could result in hypogammaglobulinemia. This might make recurrent infections more likely and call for continuing antibody replacement medication.
- Organ Toxicity: CAR T-cell therapy has the potential to harm a number of organs, including the heart, lungs, liver, and kidneys. This may lead to abnormal renal function tests, respiratory issues, heart issues, and abnormal liver function tests.
- Hemophagocytic lymphohistiocytosis (HLH): A rare but possibly fatal immunological disease called hemophagocytic lymphohistiocytosis (HLH) can develop as a result of CAR T-cell therapy. It involves the overactivation of immune cells, which causes serious organ damage and inflammation.
- Hypotension and Fluid Retention: As a result of the cytokines that CAR T cells release, some patients may develop low blood pressure (hypotension) and fluid retention. To address these symptoms, supportive measures including intravenous fluids and drugs could be required.
- Secondary Malignancies: Reports of secondary malignancies emerging following CAR T-cell therapy exist, notwithstanding their rarity. Research is currently being done on the potential for secondary malignancies and long-term hazards.
It’s important to remember that not every patient will have these side effects, and that each person’s level of sensitivity will differ. In order to minimize and minimize these potential adverse effects, the medical team closely examines patients before, during, and after CAR T-cell therapy.

You may like to read: CAR T-Cell therapy in China
Time frame
Check below the total time frame required for complete the CAR T-Cell therapy process. Although time frame depends a lot on the distance of lab from the hospital that prepared the CAR’s.
- Examination & test: one week
- Pre-treatment & T-Cell Collection: one week
- T-Cell preparation & return: two-three weeks
- 1st Effectiveness analysis: three weeks
- 2nd Effectiveness analysis: three weeks.
Total time frame: 10-12 Weeks
Cost of CAR T-Cell therapy
CAR T cell therapy is a new and potentially effective way to treat some types of cancer, especially blood cancers like leukaemia and lymphoma. But it is also known for being expensive. The cost of CAR T cell treatment depends on a number of things, such as the type of therapy used, the type of cancer being treated, and the country’s health care system.
In general, CAR T cell therapy is a complicated process that involves taking a patient’s own immune cells, changing them in a lab to make them express chimeric antigen receptors (CARs), and then putting them back into the patient to target and kill cancer cells. From collecting the cells to giving them back to the patient, the whole process needs specialised facilities, skilled medical professionals, and cutting-edge technology, all of which add to the high cost.
CAR T cell therapy can cost anywhere from tens of thousands of dollars to millions of dollars per treatment. This includes not only the costs of the therapy itself, but also the costs of pre-treatment tests, hospitalisation, tracking, and dealing with any possible side effects. Also, some patients may need more than one dose of CAR T cell therapy, which would add to the total cost.
Even though the high cost of CAR T cell therapy makes it hard for patients and healthcare systems to pay for, it is important to remember that ongoing study and progress in the field are working to make this treatment easier to get and less expensive. People are working to simplify the manufacturing process, cut costs, and look into alternative payment models to make this groundbreaking treatment more affordable and give more people access to it.
Cost of CAR T-Cell therapy in different countries:
USA – $ 500,000 – 700,000 USD
Israel – $ 75,000 – 100,000 USD
China – $ 60,000 – 80,000 USD
UK – $ 500,000 – 700,000 USD
Singapore – $ 500,000 – 700,000 USD
Australia – $ 500,000 – 700,000 USD
South-Korea – $ 500,000 – 700,000 USD
Japan – $ 500,000 – 700,000 USD

You may like to read: CAR T-Cell therapy in South-Korea
Video: CAR T-Cell therapy
Emily Whitehead – First patient to receive CAR T-Cell therapy