Unlocking the genetic code: The future of gene therapy for genetic disorders
What is gene therapy?
Gene therapy acts as the lighthouse of contemporary medicine by offering to help correct hereditary afflictions at their very roots—the genome itself. Such treatment involves the introduction of nucleic acid polymers into the cells of a patient to improve, or even inhibit, a certain disease process. Imagine a world where the occurrence of genetic disorders does not mean a life sentence but is a condition that can be treated; that is what gene therapy promises.
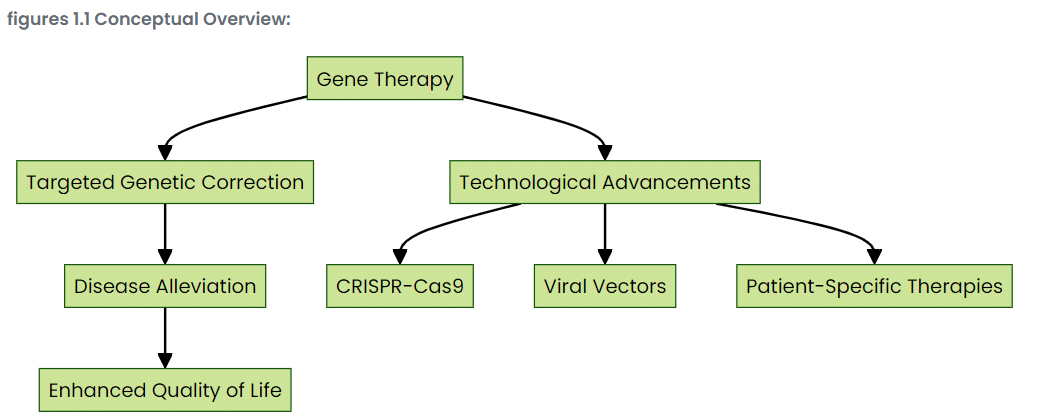
Gene therapy techniques and approaches
Well, gene therapy’s not just one single technology—rather, many technologies take part in the fight against genetic illnesses. Therapeutic genes are ferried into cells by inflamed viral vectors, like a Trojan horse. Molecular scissors extraordinaire, the CRISPR-Cas9 system snips and edits DNA with a precision that takes one’s breath away. Then there is autologous cell therapy, where a patient’s cells are modified and reinfused into the body to set the stage for self-healing.
Navigating the moral maze of gene therapy
Complex ethical questions will loom large in our future with a genetic renaissance. With this colossal power to rewrite genetic script comes related responsibility. How do we balance the potential eradication of crippling diseases against genetic discrimination or ‘designer babies? The moral compass by which gene therapy is conducted requires constant readjustment as society evolves in the face of such ground-breaking technologies.
The patient perspective: Stories of hope and resilience
On the other side of these breakthroughs in science are the very personal stories of people whose lives are hanging in the balance. The stories behind gene therapy patients are full of hope and, at the same time, vignettes to human resilience. These are the stories that underline tangible benefits from gene therapy—stories that are really colored into an extremely detailed picture of transformation in lives.
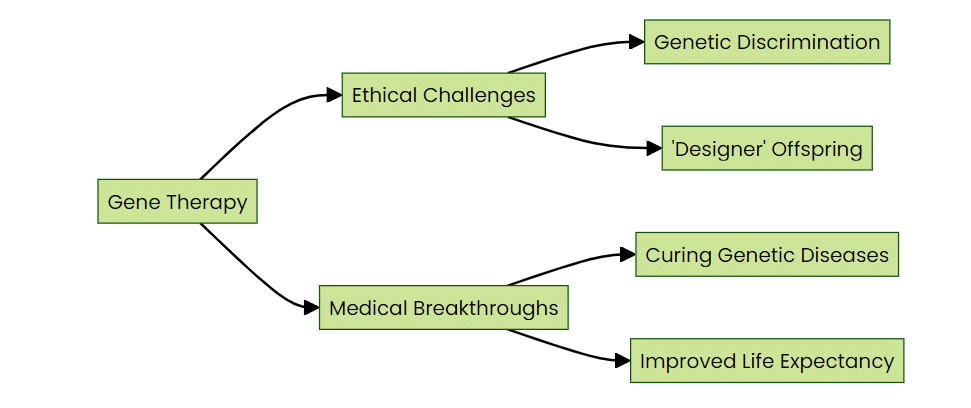
Pic. 1.2: Ethical considerations in gene therapy
The next frontier in gene therapy innovation
The trajectory of gene therapy is the epitome of innovation. Every year brings advancement that points this field toward new horizons. These are not examples of incremental upgrades but quantum leaps in biotechnology. Imagine times when genetic disorders ceased to be a life sentence and became instead a curable condition. We are right on top of such a world, for the research in the pipeline is really testing the bounds of what is possible in medicine and healing.
FAQ’s on gene therapy
- What is gene therapy?
Gene therapy involves altering genes within a patient’s cells to treat or prevent disease. - How does gene therapy work?
It works by replacing, inactivating, or introducing genes into cells, often using vectors like viruses. - What are the types of gene therapy?
There are several types, including somatic, germline, and in vivo or ex vivo therapies. - What diseases can be treated with gene therapy?
It can treat various genetic disorders, such as cystic fibrosis, hemophilia, and muscular dystrophy. - What are the risks associated with gene therapy?
Risks include immune reactions, infection from vectors, and potential long-term effects. - How is gene therapy regulated?
It’s regulated by entities like the FDA in the U.S., which assess safety and efficacy. - Can gene therapy cure genetic disorders?
While it has the potential to cure, most therapies are currently focused on treatment. - What is the future of gene therapy?
The future looks promising with ongoing research and advancements in delivery methods. - How does gene therapy impact patients’ lives?
It can significantly improve quality of life by alleviating disease symptoms or slowing progression. - What are the ethical considerations of gene therapy?
Ethical issues include concerns about consent, accessibility, and the potential for genetic enhancement.
References:
- Introduction to Gene Therapy:
- Ginn, S.L., Amaya, A.K., Alexander, I.E., Edelstein, M.L., & Abedi, M.R. (2018). Gene therapy clinical trials worldwide to 2017: An update. Journal of Gene Medicine, 20(5), e3015. doi:10.1002/jgm.3015.
- CRISPR-Cas9 Technology:
- Doudna, J.A., & Charpentier, E. (2014). The new frontier of genome engineering with CRISPR-Cas9. Science, 346(6213), 1258096. doi:10.1126/science.1258096.
- Successes in Treating Genetic Disorders:
- Frangoul, H., Altshuler, D., Cappellini, M.D., Chen, Y., Domm, J., Eustace, B.K., … & Walters, M.C. (2021). CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. New England Journal of Medicine, 384(3), 252-260. doi:10.1056/NEJMoa2031054.
- Gene Therapy for Cystic Fibrosis:
- Keown, K., Smalley, R., & Singh, M. (2021). Gene therapy for cystic fibrosis: The end of the beginning. NPJ Genomic Medicine, 6(1), 1-10. doi:10.1038/s41525-020-00161-1.
- Duchenne Muscular Dystrophy:
- Mendell, J.R., Sahenk, Z., Lehman, K., Nease, C., Lowes, L.P., Miller, N.F., … & Rodino-Klapac, L.R. (2020). Assessment of Systemic Delivery of rAAVrh74.MHCK7.micro-dystrophin in Children with Duchenne Muscular Dystrophy: A Nonrandomized Controlled Trial. JAMA Neurology, 77(9), 1122-1131. doi:10.1001/jamaneurol.2020.1484.
- Challenges and Ethical Considerations:
- Lanphier, E., Urnov, F., Haecker, S.E., Werner, M., & Smolenski, J. (2015). Don’t edit the human germ line. Nature, 519(7544), 410-411. doi:10.1038/519410a.
- Delivery Methods:
- Yin, H., Kauffman, K.J., & Anderson, D.G. (2017). Delivery technologies for genome editing. Nature Reviews Drug Discovery, 16(6), 387-399. doi:10.1038/nrd.2016.280.
- Regulatory and Safety Concerns:
- High, K.A., & Roncarolo, M.G. (2019). Gene Therapy. New England Journal of Medicine, 381(5), 455-464. doi:10.1056/NEJMra1706910.
- Advances in Gene Editing Techniques:
- Anzalone, A.V., Randolph, P.B., Davis, J.R., Sousa, A.A., Koblan, L.W., Levy, J.M., … & Liu, D.R. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature, 576(7785), 149-157. doi:10.1038/s41586-019-1711-4.
- Future Prospects and Research Directions:
- Collins, F.S., & Varmus, H. (2015). A new initiative on precision medicine. New England Journal of Medicine, 372(9), 793-795. doi:10.1056/NEJMp1500523.
Dr. Nishant Mittal is a highly accomplished researcher with over 13 years of experience in the fields of cardiovascular biology and cancer research. His career is marked by significant contributions to stem cell biology, developmental biology, and innovative research techniques.
Research Highlights
Dr. Mittal's research has focused on several key areas:
1) Cardiovascular Development and Regeneration: He studied coronary vessel development and regeneration using zebrafish models1.
2) Cancer Biology: At Dartmouth College, he developed zebrafish models for studying tumor heterogeneity and clonal evolution in pancreatic cancer.
3) Developmental Biology: His doctoral work at Keio University involved identifying and characterizing medaka fish mutants with cardiovascular defects.
4) Stem Cell Research: He investigated the effects of folic acid on mouse embryonic stem cells and worked on cryopreservation techniques for hematopoietic stem cells.
Publications and Presentations
Dr. Mittal has authored several peer-reviewed publications in reputable journals such as Scientific Reports, Cardiovascular Research, and Disease Models & Mechanisms1. He has also presented his research at numerous international conferences, including the Stanford-Weill Cornell Cardiovascular Research Symposium and the Weinstein Cardiovascular Development Conference.
In summary, Dr. Nishant Mittal is a dedicated and accomplished researcher with a strong track record in cardiovascular and cancer biology, demonstrating expertise in various model systems and a commitment to advancing scientific knowledge through innovative research approaches.
- Comments Closed
- July 18th, 2024





CRISPR-Cas9 breakthroughs, DNA repair therapies, Future of precision medicine, Gene therapy for genetic disorders, Genetic code editing, Inherited disorder cures, Next-generation gene editing, Rare disease treatment
🌟 Welcome to Beijing Biotech! 🌟
Thank you for reaching out! We are dedicated to transforming lives through advanced gene therapy solutions for sickle cell disease and thalassemia. Our team is committed to delivering cutting-edge treatments, personalized care, and support on every step of your journey to better health.
Please feel free to ask any questions or let us know how we can assist you. Together, we’re here to make a difference.
Warm regards,
The Beijing Biotech Team