Gamma Delta (γδ) T cells as a potential treatment for glioblastoma
Introduction
Despite being a minority among T cells, γδ T cells play crucial roles in immune defense against infections and in the suppression of malignancies. T cells have the potential to be a good treatment option for cancers that do not respond to standard methods because they can find tumors in different types of tissue and recognize them in different ways.
The World Health Organization has assigned the highest grade to glioblastoma (GBM), a malignant brain tumor with a very poor prognosis. There is a tumor microenvironment (TME) that weakens the immune system, and glioma stem cells hide from the immune system. These are two main reasons why immunotherapy for GBM does not work. Currently, many research groups have shown that γεT cells are a better way to treat glioblastoma (GBM). These cells have been shown to have a strong anti-tumor effect in both preclinical and clinical settings.
Nevertheless, there are still certain constraints that impede the successful utilization of γδ T cells for efficient GBM treatment. Hence, it is crucial to comprehend the specific functions of γδ T cells in the immune response to tumors and the mechanism by which the GBM tumor microenvironment suppresses these cells. This understanding is essential for the effective use of Gamma-Delta (γδ) T cell therapy for GBM.
This review gives a short summary of the functions of γε T cells in tumor immunity and looks at the newest developments and problems with using γε T cells for immunotherapy in glioblastoma (GBM). In addition, we propose potential strategies to address the constraints of γδ T cell–based immunotherapy for glioblastoma (GBM) in order to achieve effective treatment of GBM.
Gamma delta (γδ) T cells, characterized by their unique utilization of the gamma delta T cell receptor (TCR), make up around 5% of the total population of T lymphocytes. Like traditional alpha-beta T cells, gamma-delta T cells have the ability to identify specific targets and carry out direct cytotoxic activities by releasing granzymes or perforin. They also stimulate immunological responses in other cells by releasing cytokines, so contributing to the defense of the host against different diseases or malignancies.
Contrary to ab T cells, γδ T cells have the ability to identify surface molecules instead of peptides on the major histocompatibility complex (MHC). Human Vd2+ T cells specifically identify the butyrophilin family 2A1 and 3A1 complex (BTN2A1–BTN3A1 complex) connected by phosphoantigens, while Vd1+ T cells detect the MHC class I chain-related protein A.
Surface molecules are increased in the presence of infection or cellular injury, resulting in upregulation (9, 10). As a result, the detection of targets by γδ T cells through γδTCR is similar to that of pattern recognition receptors. Thus, γδ T cells serve as intermediaries between the innate and adaptive immune responses, and serve as the initial defense mechanism of the body during the early stages of infection.
Furthermore, γδ T cells have exhibited their significance in immune responses pertaining to malignancies, in addition to infection. γδ T cells exhibit not just peripheral organ localization but also circulation via the bloodstream and lymphatic system. Thus, they have crucial functions in the immune responses against tumors in solid cancers, such as lung or colorectal cancer, as well as in blood cancer. For solid tumors, a high level of γδ T cell infiltration is a positive indicator of prognosis.
Consequently, numerous research teams have examined γδ T cell–based immunotherapeutic approaches for the treatment of cancer. Due to their varied target recognition mechanism, propensity for activation through different types of stimulation, ability to carry out cytotoxic effector functions, and ability to recognize targets independently of MHC, γδ T cells have the potential to be effective immunotherapeutic agents for targeting tumors that are unresponsive to current therapeutic procedures. Consequently, numerous research teams are studying γδ T cell–based cancer therapy that focuses on different tumor models.
Glioblastoma (GBM) is a malignant brain tumor that is the most prevalent and deadly form of cancer among malignancies in the central nervous system (CNS). The absence of identifiable risk factors, along with vague symptoms, renders the early diagnosis of GBM challenging, thereby reducing the survival rate.
Several research groups have conducted thorough investigations to discover a potent treatment for GBM. Consequently, a range of mechanical, pharmacological, and immunological therapy methods have been created for GBM. While many treatments have demonstrated significant improvements in patient survival rates, a considerable number of these procedures did not yield notable outcomes.
Hence, the discovery of new therapeutic techniques is crucial for the successful management of GBM. This review will provide a concise overview of the immunologic characteristics of γδ T cells, with a specific emphasis on their involvement in anti-tumoral immune responses. Next, we will examine the present immunotherapeutic strategies used in the treatment of GBM, as well as the difficulties posed by the tumor microenvironment (TME) of GBM. In addition, we will examine the existing strategies for targeting GBM utilizing γδ T cells and the constraints associated with γδ T cell-based therapies. Ultimately, we will propose potential remedies to address the obstacles in γδ T cell–based immunotherapy for GBM.
Gamma-delta (γδ) T cells
Gamma-delta (γδ) T cells are a distinct subgroup of T cells that express the γδTCR instead of the normal alpha-beta (ab) TCR. Despite being a minority among circulating lymphocytes, γδ T cells are found in peripheral organs and barrier locations such as the skin, mucosal tract of the intestine or reproductive organs, and pulmonary tract. In fact, they make about 15–30% of the intraepithelial lymphocytes in the human gut.
Gamma-delta T cells are classified into different subsets based on their usage of Vg (in mice) or Vd (in humans), and their location is determined by the utilization of Vg or Vd. Γδ T cells in mice express Vg1 or Vg4 (according to Tonegawa nomenclature) and can be found in the bloodstream. Vg5 is specifically found in the skin, Vg6 is found in the dermis and meninges, and Vg7 is found in the gut. Human Vd2+ γδ T cells are found in the bloodstream, while Vd1+ and Vd3+ γδ T cells exhibit characteristics of resident cells.
Despite their low representation within the T cell population, γδ T cells play a crucial role in the immune system by exerting different effector functions and localizing to specific tissues. They serve as a frontline defensive mechanism by immediately inhibiting pathogenic infections and functioning as both innate and adaptive immune cells.
γδ T cells have a broader range of recognition for different surface molecules compared to traditional αβ T cells, which specifically detect peptides presented on the major histocompatibility complex (MHC). As an illustration, human Vd1+ γδ T cells have the ability to identify and bind to the CD1d molecule. Similarly, Vg8Vd3+ T cells can recognize stress-induced annexin A2, whereas Vg9Vd1+ T cells are capable of recognizing ephrin type-A receptor 2 activation by AMP-activated protein kinase.
Furthermore, Vg9Vd2+ T lymphocytes present in the peripheral blood have the ability to identify the BTN2A1-BTN3A1 complex when phospho antigens are present. γδ TCRs exhibit similarity to pattern recognition receptors due to their ability to identify stress-induced chemicals present on the surface of target cells.
Thus, γδ T cells exhibit invariant or semi variant markers, in contrast to ab T cells, that must recognize many peptides. Consequently, TCR diversity is of utmost importance. Furthermore, γδ T cells possess the ability to identify a wide range of surface chemicals through NK receptors (NKRs) in addition to the γδTCR. These cells also demonstrate enhanced effector capabilities when the γδ TCR is engaged.
Furthermore, γδ T cells can exhibit cytolytic activity by means of death ligands such as Fasligand or TRAIL, in addition to their ability to recognize and target cells through γδTCR and NKR-mediated mechanisms. Γδ T cells have diverse target identification methods and are crucial in providing initial protection for different tissues.
γδ T cells possess diverse effector capabilities and exhibit similar effector actions to typical αβ T cells. For instance, gamma-delta T cells exert cytotoxic effects on target cells through the synthesis of granzyme and perforin, much like CD8+ T cells with cytotoxic activity. In addition, gamma delta (γδ) T cells produce a range of cytokines, such as IFNg and TNFa, indicating their ability to influence the immune system by producing cytokines.
Moreover, like effector CD4+ T cells, γδ T cells undergo polarization into specific subtypes and simultaneously secrete cytokines that impact the nearby immunological milieu. Within the population of mice γδ T cells, there is a distinction between the development and functions of IL-17-producing γδ T cells and IFN-g-producing γδ T cells in the thymus.
Human Vg9Vd2+ T cells exhibit functional adaptability based on their cytokine exposure following TCR stimulation. Γδ T cells possess functional plasticity, allowing them to act as versatile effectors that can have both beneficial and detrimental impacts in various disease states, such as cancer.
Role of γδ T cells in tumor quashing
The diverse functions of γδ T cells in tumor settings include their well-studied tumor-suppressive roles. These roles have been extensively investigated by various research groups due to the high cytotoxicity, versatile effector function, and distinctive tissue localization of γδ T cells. Additionally, the presence of γδ T cells is considered a favorable prognostic indicator for all solid tumor types.
Liu et al. demonstrated in a mouse model of prostate cancer that the absence of γδ T cells led to substantial tumor growth, while the introduction of γδ T cells through adoptive transfer dramatically decreased the amount of tumor present. In addition, γδ T cells exhibited enhanced tumor control in comparison to an equivalent amount of standard ab T cells, indicating that γδ T cells possess stronger tumor suppression and target-lysing capabilities in comparison to conventional T cells lacking tumor specificity.
Similarly, in the azoxymethane-induced colorectal cancer model, mice without γδ T cells exhibited a greater tumor incidence compared to mice lacking ab T cells. This indicates that γδ T cells can function as a primary tumor suppressor.
Furthermore, in the context of chemically generated skin cancer, the elimination of γδ T cells resulted in a notable increase in tumor growth, whereas the removal of ab T cells had no impact on the formation and growth of tumors. Thus, γδ T cells function as tumor suppressors in multiple organs, such as the skin and colon. Human γδ T cells have also exhibited anti-tumor functionality, in addition to the research conducted on mice γδ T cells.
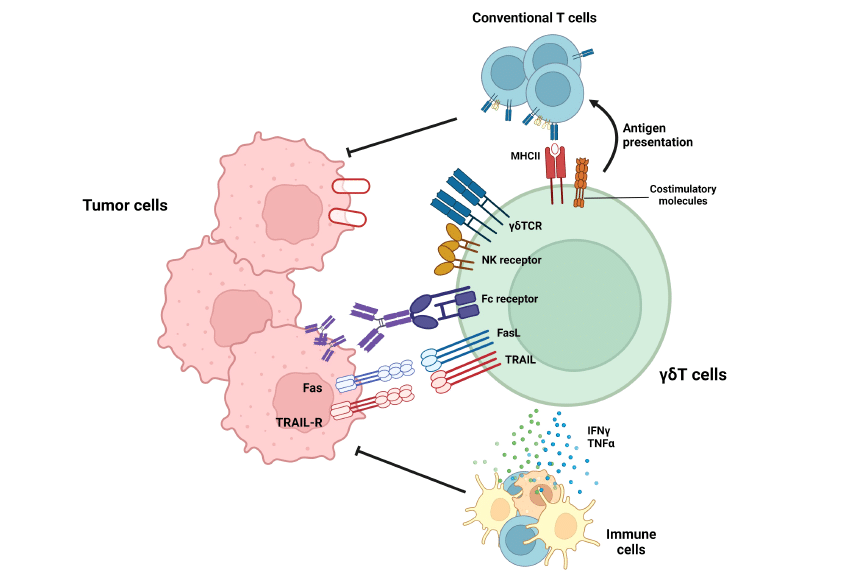
FIGURE 1
Roles of gd T cells in tumor suppression. gd T cells exert anti-tumoral immune responses by diverse mechanisms. By recognizing target molecules via gdTCR and NKG2D, gd T cells can lyse tumor cells. In addition, Vd2+ gd T cells can eliminate tumor cells by antibody-dependent cell-mediated cytotoxicity (ADCC) in a CD16-dependent manner. Furthermore, gd T cells can suppress tumor cells by death ligands, such as TRAIL or Fas ligands. In addition to these direct killings, gd T cells can indirectly suppress tumor cells by activating T cells via working as antigen-presenting cells (APCs) or facilitating other immune cells via pro-inflammatory cytokine secretion.
Figure 1 provides a summary of the anti-tumoral activities carried out by γδ T cells. Human Vd2+ γδ T lymphocytes can exhibit anti-tumoral effects via binding to the BTN2A1-BTN3A1 complex in the presence of phosphoantigens (61). Furthermore, Vd2+ γδ T cells exhibit cytolytic activity through NKG2D-mediated identification of targets, in addition to their cytotoxicity mediated by γδTCR (62).
In addition, it has been observed that human Vd2+ γδ T cells had the ability to kill tumor cells by antibody-dependent cell-mediated cytotoxicity, whereas Vd1+ γδ T cells do not possess this capability. Furthermore, the extent of cytotoxicity exhibited by Vd2+ γδ T cells was directly proportional to the overexpression of CD16 (63). γδ T cells not only control tumor growth through their lethal effector function, but also modulate the activity of other immune cells.
Activated γδ T cells have an increase in MHC-II and other co-stimulatory markers (CD40, CD80, and CD86) unlike ab T cells. Additionally, they have the ability to activate traditional T cells (64). γδ T cells possess both high cytotoxicity and the ability to eliminate tumor cells. Furthermore, they can also deliver the tumor antigen to conventional T cells, so promoting a systemic immune response against tumor cells.
Furthermore, γδ T cells have the ability to enhance the activity of dendritic cells, therefore promoting the process of antigen presentation and activation of conventional T cells. To summarize, γδ T cells have the ability to effectively destroy tumor cells, disperse the tumor antigen, and support adaptive and systemic immune responses against malignancies. Thus, γδ T cells have the potential to enhance existing anti-tumor immunotherapy methods.
Consequently, numerous research teams have broadened the use of γδ T cells by examining their functions and abilities to combat other forms of cancer. They have also endeavored to cure tumors that are unresponsive to existing therapeutic methods such immune checkpoint inhibitors. One instance of these cancer kinds is GBM, which is a malignant brain cancer that exhibits
Restricted therapeutic efficacy of immune checkpoint inhibitors. Recently, multiple research groups have shown the significance of γδ T cells in the suppression of glioma. Thus, γδ T cells possess the capacity to serve as a potent therapeutic agent for GBM. Nevertheless, there are certain constraints that hinder the maximum effectiveness of γδ T cells in the GBM TME.
Consequently, we will now proceed to address the overall context and contemporary therapeutic approaches that specifically target GBM. Additionally, this study will examine the latest developments and constraints in the use of γδ T cells for treating GBM. Ultimately, we will propose various approaches to address the constraints of γδ T cells. GBM immunotherapy targets cells in glioblastoma.
Current advances in γδ T cell-mediated GBM immunotherapy
The robust anti-tumor capabilities of γδ T lymphocytes in GBM have prompted extensive investigation at both the preclinical and clinical stages. Park et al. established that the augmentation of γδ T lymphocytes serves as a favorable indicator for survival in both mice and humans. Nevertheless, the actions of γδ T cells in the tumor microenvironment (TME) are inhibited by extreme hypoxia. Consequently, γδ T cells decrease the expression of NKG2D, leading to the inhibition of their ability to identify targets and carry out their activities as effectors. Thus, the functioning of γδ T cells was restored by treating tumor hypoxia with metformin.
Lee et al. discovered that Vg9Jg2-Vd2 T cells had a tendency to enter the GBM TME more than other cells. This suggests that human γδ T cells play a role in suppressing tumors in a living organism (69). Using an in-vitro cytotoxicity paradigm, it was observed that γδ T cells generated from human peripheral blood mononuclear cells (PBMCs) exhibited more cytotoxicity against the U251MG human glioma cell line as compared to ab T cells. Furthermore, γδ T cells generated from human peripheral blood mononuclear cells (PBMCs) did not exhibit cytotoxicity against non-tumor cells, such as primary human astrocytes.
The efficacy of γδ T cells in GBM treatment is further demonstrated by their capacity to inhibit GSCs, which play a crucial role in tumor development, maintenance, metastasis, and resistance to conventional therapy. GSCs escape detection by the immune system by the downregulation of MHC class I molecules and the antigen processing machinery, therefore avoiding the immunological response mediated by CD8 T cells. However, γδ T lymphocytes have the ability to specifically attack GSCs.
Jarry et al. introduced primary glioblastoma multiforme (GBM) cells that contained a high proportion of glioblastoma stem cells (GSCs) (about 25%) into the brains of mice that had a fully functioning immune system (NSG mice). Subsequently, bromohydrin pyrophosphate–activated human Vg9Vd2+ T cells were administered via injection at the tumor site, resulting in effective suppression of tumor growth when combined with zoledronate (128). The exceptional targeting capability of γδ T lymphocytes is derived from their minimal activation threshold. CD8 T cells necessitate both NKG2D and TCR signaling for activation, but γδ T cells can be activated only by NKG2D.
Thus, γδ T cells exhibit enhanced activation in the absence of TCR interaction, rendering it challenging for tumor cells to elude detection by γδ T cells. Based on the positive effects of these effector activities, Choi et al. demonstrated that transferring human Vg9Vd2+ T cells directly into tumors greatly enhanced the survival rate of mice that had been treated with the U87 human glioma cell line. When examining the interaction between γδ T cells and a sample collected from a glioma patient, it was observed that Vg9Vd2+ T cells exhibited cytotoxicity through the DNAM1 pathway.
This suggests that γδ T cells may have a mechanism for killing tumor cells in glioblastoma (GBM). Nevertheless, clinical investigations involving γδ T cells have yielded unsatisfactory outcomes in different tumor scenarios, with only one ongoing clinical trial specifically focusing on the use of γδ T cells to target GBM (ClinicalTrials.gov Identifier: NCT04165941).
Although γδ T cells did not induce severe toxicity following in-vitro expansion and subsequent adoptive transfer, their therapeutic impact was only moderate. Despite the potential of γδ T cells as a kind of immunotherapy for treating many malignancies, including GBM, there are several challenges that need to be addressed in order to effectively deploy γδ T cells in a therapeutic context.
Dr. Nishant Mittal is a highly accomplished researcher with over 13 years of experience in the fields of cardiovascular biology and cancer research. His career is marked by significant contributions to stem cell biology, developmental biology, and innovative research techniques.
Research Highlights
Dr. Mittal's research has focused on several key areas:
1) Cardiovascular Development and Regeneration: He studied coronary vessel development and regeneration using zebrafish models1.
2) Cancer Biology: At Dartmouth College, he developed zebrafish models for studying tumor heterogeneity and clonal evolution in pancreatic cancer.
3) Developmental Biology: His doctoral work at Keio University involved identifying and characterizing medaka fish mutants with cardiovascular defects.
4) Stem Cell Research: He investigated the effects of folic acid on mouse embryonic stem cells and worked on cryopreservation techniques for hematopoietic stem cells.
Publications and Presentations
Dr. Mittal has authored several peer-reviewed publications in reputable journals such as Scientific Reports, Cardiovascular Research, and Disease Models & Mechanisms1. He has also presented his research at numerous international conferences, including the Stanford-Weill Cornell Cardiovascular Research Symposium and the Weinstein Cardiovascular Development Conference.
In summary, Dr. Nishant Mittal is a dedicated and accomplished researcher with a strong track record in cardiovascular and cancer biology, demonstrating expertise in various model systems and a commitment to advancing scientific knowledge through innovative research approaches.
- Nishant Mittal (PhD)https://cancerfax.com/author/nishantm/
- Nishant Mittal (PhD)https://cancerfax.com/author/nishantm/
- Nishant Mittal (PhD)https://cancerfax.com/author/nishantm/
- Nishant Mittal (PhD)https://cancerfax.com/author/nishantm/
Related Posts
- Comments Closed
- August 25th, 2024


- Datopotamab deruxtecan-dlnk is approved by the USFDA for EGFR-mutated non-small cell lung cancer
- Tafasitamab-cxix is approved by the USFDA for relapsed or refractory follicular lymphoma
- PiggyBac Transposon System: A Revolutionary Tool in Cancer Gene Therapy
- Breakthrough Treatments for Advanced Breast Cancer in 2025
- Neoadjuvant and adjuvant pembrolizumab is approved by the USFDA for resectable locally advanced head and neck squamous cell carcinoma
- Mitomycin intravesical solution is approved by the USFDA for recurrent low-grade intermediate-risk non-muscle invasive bladder cancer
- Taletrectinib is approved by the USFDA for ROS1-positive non-small cell lung cancer
- Darolutamide is approved by the USFDA for metastatic castration-sensitive prostate cancer
- Atezolizumab Plus Chemotherapy Improves Survival in Advanced-Stage Small-Cell Lung Cancer: Insights from the IMpower133 Study
- Satri-cel CAR T-Cell Therapy: A New Era in Gastric Cancer Treatment
- AI & Technology (12)
- Aids cancer (4)
- Anal cancer (9)
- Appendix cancer (3)
- Basal cell carcinoma (1)
- Bile duct cancer (7)
- Biotech Innovations (19)
- Bladder cancer (12)
- Blood cancer (60)
- Bone cancer (12)
- Bone marrow transplant (47)
- Brain Cancer (1)
- Breakthrough Research (17)
- Breast Cancer (53)
- Cancer Guides (10)
- Cancer News and Updates (54)
- Cancer Treatment Abroad (286)
- Cancer treatment in China (316)
- Cancer Treatments (12)
- Cancer Types (5)
- Cancer Warriors (1)
- CAR T Protocols (2)
- CAR T-Cell therapy (135)
- Cervical cancer (40)
- Chemotherapy (55)
- Childhood cancer (2)
- Cholangiocarcinoma (3)
- Clinical trials (15)
- Colon cancer (96)
- Diagnosis & Staging (4)
- Doctors & Researchers (76)
- Drug Approvals (100)
- Drugs (80)
- Endometrial cancer (10)
- Esophageal cancer (15)
- Eye cancer (9)
- For Doctors and Researchers (12)
- Gall bladder cancer (3)
- Gastric cancer (29)
- Gene therapy (5)
- Glioblastoma (7)
- Glioma (10)
- Global Trial News (5)
- Gynecological cancer (2)
- Head and neck cancer (19)
- Hemato-Oncologist (1)
- Hematological Disorders (52)
- Hospital Reviews (3)
- How to Participate (6)
- Immunotherapy (34)
- Kidney cancer (10)
- Laryngeal cancer (1)
- Leukemia (49)
- Liver cancer (101)
- Lung cancer (82)
- Lymphoma (52)
- MDS (2)
- Melanoma (9)
- Merkel cell carcinoma (1)
- Mesothelioma (5)
- Myeloma (25)
- Myths vs Facts (5)
- Neuroblastoma (7)
- NK-Cell therapy (13)
- Nutrition (1)
- Ongoing Trials (11)
- Oral cancer (12)
- Ovarian Cancer (14)
- Pancreatic cancer (43)
- Paraganglioma (6)
- Patient Testimonials (1)
- Penile cancer (1)
- Prostrate cancer (11)
- Proton therapy (28)
- Radiotherapy (56)
- Recovery Tips (2)
- Rectal cancer (58)
- Research Insights (8)
- Sarcoma (14)
- Skin Cancer (13)
- Spine surgery (24)
- Stomach cancer (40)
- Success Stories (1)
- Surgery (102)
- Systemic mastocytosis (1)
- T Cell immunotherapy (7)
- Targeted therapy (9)
- Testicular cancer (5)
- Thoracic surgery (2)
- Throat cancer (6)
- Thyroid Cancer (15)
- Treatment Cost (1)
- Treatment in China (969)
- Treatment in India (1,273)
- Treatment in Israel (652)
- Treatment in Malaysia (425)
- Treatment in Singapore (321)
- Treatment in South Korea (305)
- Treatment in Thailand (291)
- Treatment in Turkey (272)
- Treatment Planning (151)
- Trial Results (2)
- Uncategorized (105)
- Urethral cancer (9)
- Urosurgery (14)
- Uterine cancer (4)
- Vaginal cancer (6)
- Vascular cancer (5)
- Vulvar cancer (1)


Brain tumor treatment, CAR-T alternatives, Cellular therapy for GBM, Gamma Delta T-cell clinical trials, Gamma Delta T-cells glioblastoma, Glioblastoma immunotherapy, Novel glioblastoma treatments, γδ T-cell therapy
CancerFax is the most trusted online platform dedicated to connecting individuals facing advanced-stage cancer with groundbreaking cell therapies.
Send your medical reports and get a free analysis.
🌟 Join us in the fight against cancer! 🌟
Привет,
CancerFax — это самая надежная онлайн-платформа, призванная предоставить людям, столкнувшимся с раком на поздних стадиях, доступ к революционным клеточным методам лечения.
Отправьте свои медицинские заключения и получите бесплатный анализ.
🌟 Присоединяйтесь к нам в борьбе с раком! 🌟