Understanding Cytokine Release Syndrome: Causes, Symptoms, and Treatment
Introduction
Cytokine Release Syndrome (CRS) is an immune system overreaction triggered by therapies like immunotherapy or CAR-T cell treatment. It prompts an influx of cytokines, causing symptoms ranging from fever to potentially fatal complications like organ failure. Managing CRS involves vigilant monitoring and may require interventions to mitigate its severity.
Infections, autoimmune diseases, and immunotherapy are among the many potential causes of cytokine release syndrome (CRS), a complicated immune system reaction. Symptoms of chronic inflammatory syndrome (CRS) can range from moderate pain to potentially fatal problems caused by an overproduction of cytokines, which are tiny proteins that communicate between cells. Because of its link to cutting-edge cancer treatments, especially chimeric antigen receptor (CAR) T-cell therapy, CRS has been in the spotlight recently. The goals of this article are to discuss CRS and its symptoms, as well as its diagnosis and possible treatments.
Why do people experience cytokine release syndrome?
While many things can set off CRS, immunotherapy, and specifically CAR T-cell therapy, is by far the most common culprit. The goal of chimeric antigen receptor (CAR) T-cell therapy is to improve the efficiency with which a patient’s immune system attacks cancer cells. On the other hand, this procedure has the potential to trigger T cell activation and proliferation, which in turn might cause an increase in cytokine production and CRS.
Immunotherapy isn’t the only thing that might cause CRS; infections (whether bacterial or viral) and autoimmune diseases (when the immune system assaults healthy tissues) are additional potential causes.
Cytokine release syndrome: signs and symptoms
Fever, lethargy, headache, nausea, vomiting, diarrhea, muscular aches, fast heart rate, low blood pressure, and trouble breathing are some of the symptoms of chronic rhinovirus syndrome, the severity of which can vary greatly. When CRS worsens to the point where two or more organ systems fail, a potentially fatal illness known as multi-organ dysfunction syndrome (MODS) can develop.
The severity of CRS symptoms frequently correlates with the degree to which the underlying cause activates the immune response. As an example, CRS is commonly seen in patients undergoing CAR T-cell therapy; symptoms such as high fever, hypotension, and respiratory distress may appear soon after treatment.
How do you determine if you have cytokine release syndrome?
The symptoms of CRS are not always easy to identify because they can be similar to those of other diseases or reactions, such as sepsis or allergies. Medical professionals usually diagnose CRS and rule out other potential causes of symptoms by combining clinical evaluation with laboratory tests, imaging scans, and blood tests to measure cytokine levels.
Many organizations have established grading systems for CAR T-cell therapy-specific CRS, using clinical and laboratory characteristics to determine its severity. These systems include the Lee criteria and the Common Terminology Criteria for Adverse Events (CTCAE).
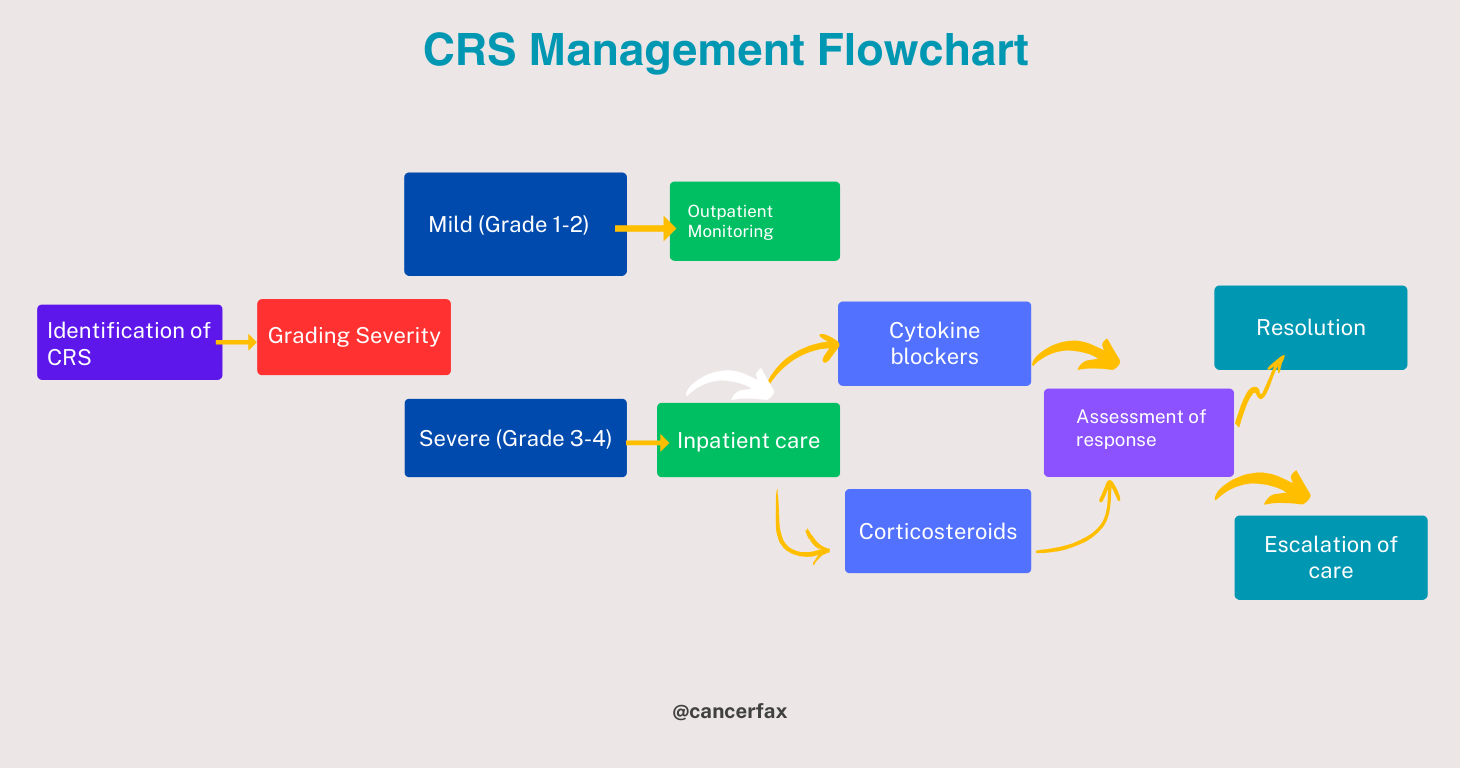
Cytokine release syndrome treatment
The severity and etiology of CRS dictate its treatment. Supportive therapy, including hydration, temperature reducers, and nausea and vomiting suppressants, may be enough in moderate cases. More drastic measures, though, may be required in the most serious instances.
The immune response is suppressed and symptoms are alleviated with the use of corticosteroids in cases of CRS related to immunotherapy. Tocilizumab, a monoclonal antibody that targets the interleukin-6 (IL-6) receptor, has also shown promising results when corticosteroids alone are not enough to ease the symptoms of CRS.
In the worst cases of CRS with unstable blood flow or organ dysfunction, critical care may include renal replacement therapy, mechanical ventilation, vasopressor support, and other types of care.
Cytokine release syndrome prevention
The prevention of CRS is still a major obstacle, especially when it comes to new immunotherapies, which provide an inherent risk of CRS due to their therapeutic mechanism. To counteract this risk without compromising the effectiveness of these cutting-edge treatments, researchers are hard at work.
To lessen the likelihood of CRS without sacrificing therapeutic effectiveness, one strategy is to optimize the timing and dosage of immunotherapy. Also, in order to better monitor and intervene early with individuals who may be at a higher risk of developing CRS, researchers are looking into predictive biomarkers.
Summary
Infections, autoimmune diseases, and even some medical therapies, including immunotherapy, can set off the complicated immune system reaction known as cytokine release syndrome. The overproduction of cytokines is a hallmark of chronic inflammatory syndrome (CRS), which can cause anything from moderate pain to potentially fatal consequences. To reduce the morbidity and mortality caused by CRS, it is crucial to identify and treat the condition early on. Improving outcomes for patients affected by CRS will require ongoing research into the underlying causes of the disease and the development of tailored medicines.
Dr. Nishant Mittal is a highly accomplished researcher with over 13 years of experience in the fields of cardiovascular biology and cancer research. His career is marked by significant contributions to stem cell biology, developmental biology, and innovative research techniques.
Research Highlights
Dr. Mittal's research has focused on several key areas:
1) Cardiovascular Development and Regeneration: He studied coronary vessel development and regeneration using zebrafish models1.
2) Cancer Biology: At Dartmouth College, he developed zebrafish models for studying tumor heterogeneity and clonal evolution in pancreatic cancer.
3) Developmental Biology: His doctoral work at Keio University involved identifying and characterizing medaka fish mutants with cardiovascular defects.
4) Stem Cell Research: He investigated the effects of folic acid on mouse embryonic stem cells and worked on cryopreservation techniques for hematopoietic stem cells.
Publications and Presentations
Dr. Mittal has authored several peer-reviewed publications in reputable journals such as Scientific Reports, Cardiovascular Research, and Disease Models & Mechanisms1. He has also presented his research at numerous international conferences, including the Stanford-Weill Cornell Cardiovascular Research Symposium and the Weinstein Cardiovascular Development Conference.
In summary, Dr. Nishant Mittal is a dedicated and accomplished researcher with a strong track record in cardiovascular and cancer biology, demonstrating expertise in various model systems and a commitment to advancing scientific knowledge through innovative research approaches.
- Comments Closed
- April 29th, 2024






Cancer treatment toxicities, CAR-T therapy side effects, CRS management guidelines, CRS symptoms, Cytokine release mechanism, Cytokine storm treatment, Immunotherapy complications, Tocilizumab for CRS
CancerFax is the most trusted online platform dedicated to connecting individuals facing advanced-stage cancer with groundbreaking cell therapies.
Send your medical reports and get a free analysis.
🌟 Join us in the fight against cancer! 🌟
Привет,
CancerFax — это самая надежная онлайн-платформа, призванная предоставить людям, столкнувшимся с раком на поздних стадиях, доступ к революционным клеточным методам лечения.
Отправьте свои медицинские заключения и получите бесплатный анализ.
🌟 Присоединяйтесь к нам в борьбе с раком! 🌟