May 2022: T-cell therapy has begun a vigorous attack on digestive system malignancies, and T-cell immunotherapy has triumphed over refractory tumours like gastric cancer, liver cancer, and pancreatic cancer. Some people believe that digestive tract malignant tumours are eaten. Although exaggerated, it cannot be denied that many malignant tumours of the digestive system are inextricably linked to “feeding.” Five of the ten high-incidence malignancies in my country are linked to “eating,” according to the “2017 China Cancer Registration Annual Report.” Gastric cancer, colorectal cancer, liver cancer, esophageal cancer, and pancreatic cancer are all mentioned as digestive tract tumours that are eaten, accounting for 43.3 percent of total cancer incidence and nearly half of the country’s population. In medical terms, all tumours of the digestive tract, including the liver and pancreas, are called digestive tract tumours; in a strict sense, digestive tract tumours are gastrointestinal tumours, which include gastric cancer and intestinal cancer. The most frequent malignant tumour disease.
According to the National Cancer Registry’s most recent data, stomach cancer is the second most common malignant tumour in the same time period. The general prognosis is poor; the 5-year survival rate is about 10% to 30%, and patients with advanced gastric cancer have a 5-year survival rate of less than 10%. The findings of studies into first-line treatment for advanced gastric cancer demonstrate that overall survival (OS) is steadily improving, although the median Bit survival remains about 12 months. Immunotherapy has been widely used around the world to enhance the survival rate of stomach cancer patients. Some tumour markers for gastric cancer have been developed and examined extensively. CAR-T treatment for stomach cancer is making progress in research. CT041 is Claudin18.2’s target. Claudin18.2 (CLDN18.2) is a stomach-specific membrane protein that could be used to treat gastric cancer and other cancers. Chinese researchers used this information to create the world’s first CAR-T cells that target Claudin18.2. CT041 was announced at the 2019 ASCO annual meeting as the world’s first CAR-T cell targeting Claudin18.2. The entire objective response rate at the time was 33.3 percent, which was already incredible to the rest of the globe. The frosting on the cake is probably the more significant curative effect! This Clinical evidence suggests that the treatment of digestive system malignancies has a bright future! The research findings of Keji Pharmaceutical’s CAR-T cell product CT041 in the treatment of digestive system malignancies were published in the top international publication “Nature Medicine” on May 9, 2022, making it the first time a prominent academic journal has published such findings. A clinical trial using a small number of CAR-T cells to treat solid tumours! 37 individuals with advanced gastrointestinal cancers with positive CLDN18 as of April 8, 2021. 28 gastric cancer/gastroesophageal junction cancer, 5 pancreatic cancer, and 4 other solid tumours were included in the study. The median number of metastatic organs was three, and 84 percent of patients had had at least two previous rounds of therapy.
The research data is outstanding
1. The objective response rate of all patients was 48.6%, and the disease control rate was 73%; the overall objective response rate of all gastric cancer patients was 57.1%.
2. Patients with gastric cancer who have failed at least 2 lines of therapy in the past: the objective response rate was 61.1%, and the disease control rate was 83.3%.
3. And overall well tolerated!
As of March 3, 2022, CT041 has become the world’s first and only CAR-T cell product candidate for the treatment of solid tumors that has entered a confirmatory phase II clinical trial. This is a milestone for CAR-T to conquer the field of solid tumors.
There are also a number of CAR-T cell treatments that target CLDN18.2. Legend Bio’s LB-1904, for example, has entered Phase I clinical trials for the treatment of gastric or pancreatic cancer. Legend Bio has also released LCAR-C18S, a Claudin18.2-targeting CAR-T cell preparation for the treatment of advanced gastric cancer, and is presently enrolling patients for clinical studies. B-cell lymphoma, T-cell lymphoma, T-cell leukaemia (T-ALL), acute leukaemia, non-lymphoma, Hodgkin’s liver cancer, gastric cancer, prostate cancer, thyroid cancer, and other tumours are all being recruited right now! You can submit the pathology report, treatment experience, and discharge summary to the Cancer Free Home Medical Department for preliminary evaluation if you wish to see if your condition is suitable for CAR-T therapy.
TCR-T cell therapy has been licenced for clinical usage, opening up the possibility of a novel treatment option for HBV-related hepatocellular cancer
Domestic discoveries and developments are currently being made in addition to the worldwide TCR-T research and development and clinical validation stage. The clinical application of “SCG101 autologous T cell injection,” a TCR-T targeting particular hepatitis B virus (HBV) epitopes, can be queried on the official website of CDE (Center for Drug Evaluation) on December 22, 2021. Cell therapy could be a novel treatment option for HBV-related hepatocellular cancer (HCC).
Cancer patients who get TCR-T treatment experience complete remission
ADP-A2AFP, a new T-cell-based TCR-T therapy for liver cancer, caused quite a stir at the 2020 International Liver Congress (ILC), and also achieved significant advancements in new liver cancer treatments. One patient had a complete remission (CR) in the cancer cell process, while the remainder of the patients had varied degrees of alpha-fetoprotein (AFP) reduction, indicating that the study had advanced. It also demonstrates that the therapy is successful in treating advanced liver cancer.
So is there any chance to try TCR-T therapy?
Currently, a TCR-T therapy is being developed to recruit liver cancer patients for clinical trials. Patients who want to participate can consult the Cancer Free Home Medical Department for detailed admission criteria.
Fight against pancreatic cancer treatment
The fight against cancer’s king has always been a hot topic. Pancreatic cancer is deserving of the title of “king of cancer.” It’s a cancerous tumour that starts in the pancreatic ductal epithelium and acinar cells. It has a high malignancy level, a slow onset, difficult early detection, rapid progression, and a short survival duration. It is one of the cancers with the poorest prognosis.
Only 20% of pancreatic cancer patients are discovered in the early or intermediate stages, which implies that around 80% of patients have little chance of being treated, and the 5-year survival rate is only 10%. The mortality rate of pancreatic cancer is expected to be second only to lung cancer in 2030. This is clearly a “death sentence” in the eyes of many people, and the countdown to life begins.
01. Transforming special CAR-T technology to completely disappear advanced pancreatic cancer lesions
On July 29, the internationally known magazine “Journal of Hematology & Oncology” released a clinical report by Chinese medical researchers on the successful transformation of CAR-T technology. The researchers added interleukin 7 (IL-7) and chemokine CCL19 to make CAR-T cells have a greater ability to enter the tumour. -3 (GPC3) and mesothelin (MSLN) to make CAR-T cells have a better ability to enter the tumour. One of them, an advanced pancreatic cancer patient, received the most successful CAR-T therapy by intravenous infusion. The lesions in the entire body virtually gone after treatment. Typical scenarios The patient GD-G/M-005 had advanced pancreatic cancer with MSLN expression. Regional lymph node metastases (24 mm) developed in this patient (Panel c). Anti-MSLN-7 19 CAR-T cells were given through the hepatic artery for the first time that night, with a high temperature but no cytokine release syndrome (CRS) or neurotoxicity. Every 1–2 months, he received an intravenous infusion of anti-MSLN-719 CAR-T cells. CT staging revealed that he had achieved complete remission after 240 days of treatment, with the lymph node measured at 8.3 mm and no further enlarged lymph nodes (Figure C). At this point, the patient’s lesions had entirely vanished and he was back to normal.
Data of improved GoCAR-T technology is amazing
Bellicum Pharmaceuticals reported data from a Phase 1/2 clinical trial in patients with PSCA-expressing metastatic pancreatic cancer at the ASCO Annual Meeting in June 2019. The company’s new and improved GoCAR-T technology, BPX-601, offers favourable safety and activity data. BPX-601 has been well tolerated so far, with no dose-limiting toxicities reported. The majority of BPX-601 side effects were mild to moderate, and they went away with or without supportive care. Despite a cautious clinical trial design, early in the investigation, evidence of stimulating biological activity was discovered! Eight (62%) of the 13 patients evaluated for effectiveness had stable disease, while three individuals exhibited tumour shrinkage ranging from 10% to 24%.
In addition to the international CAR-T breakthrough clinical studies reported above, the current clinical trial targets of CAR-T therapy for pancreatic cancer include CD133, EGFR, EpCAM, MUC1, CEA, and HER2. If you want to know more about the clinical research of CAR-T solid tumors, please continue to pay attention to the cancer-free home.
Cancer with a higher degree of malignancy – colorectal cancer
According to the 2018 China Cancer Statistics Report, colorectal cancer was the third and fifth most common cancer in my country, with 376,000 new cases and 191,000 deaths, respectively. Unfortunately, most patients are in the middle or late stages of their illness when they are diagnosed. Surgery is only employed as a palliative treatment approach for patients, or to eliminate symptoms that have a bigger impact on patients, and it is primarily adjuvant therapy, with an unfavourable prognosis. Researchers have increasingly shifted their focus to the popular therapy in recent years—CAR-T therapy—since tumour immunotherapy has become the fourth cancer treatment technique. The editor has identified anti-4-1BB (ANTI-4-1BB), carcinoembryonic antigen (CEA), GUCY2C, TAG-72, EpCAM, Epiglin 40 (EGP40), NKG2D, HER-2, recombinant human interferon alpha/beta receptor 1 (IFNAR1), prominin-1 (CD133), epiglin 2 (EGP2) as promising targets of CAR-T cell treatment in colorectal (EGP-2). Among them, GUCY2C, an effective target for colorectal cancer, is a recent research hotspot. China has a small CAR-T study on 7 patients, targeting colon cancer marker GUCY2C. The study found that 2 to 3 patients had partial responses and stable disease.
Cholangiocarcinoma or bile-duct cancer
Cholangiocarcinoma is one of the most deadly cancers of the bile duct mucosal epithelium, accounting for around 3% of all digestive system tumours, peaking at 70 years of age and affecting slightly more males than women. Because there are so many cholangiocarcinoma patients in China, cholangiocarcinoma research is particularly important in my nation. Surgical resection is the best treatment for all cholangiocarcinomas, however only 10% of patients are eligible for surgery due to the difficulties of early diagnosis. Patients with cholangiocarcinoma following surgery have a 5-year survival rate of about 5%, and patients with unresectable cholangiocarcinoma have a survival time of less than a year. Cholangiocarcinoma’s clinical chemotherapeutic result isn’t great, thus new clinical research on CAR-T targeted therapy for advanced cholangiocarcinoma is exciting. A 52-year-old female patient with advanced surgically unresectable metastatic cholangiocarcinoma who declined chemoradiotherapy underwent CAR-T cocktail immunotherapy, according to a recent clinical trial research. EGFR and CD133 were the targets of CAR-T cells. After CAR-T-EGFR treatment, the patient had an 8.5-month partial response, and after CAR-T-CD133 treatment, the patient had a 4.5-month partial response.
CAR T-Cell therapy
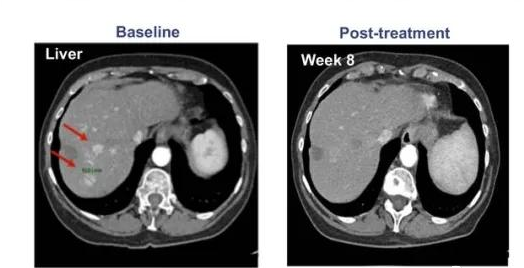
Professor Han Weidong’s team from the PLA General Hospital published preliminary results of using EGFR CAR-T technology to treat biliary tract malignancies in the internationally known publication “Clinical Cancer Research” in March 2018. A total of 19 patients with unresectable biliary tract malignant tumours with strong EGFR positive (>50 percent of cancer cells express EGFR) were included in this study, including 14 cases of cholangiocarcinoma and 5 cases of gallbladder cancer among them. The study’s findings revealed that 17 patients could be assessed, with one patient with cholangiocarcinoma having their tumour totally gone. The therapeutic effect has been maintained for 22 months, with no evidence of illness return. The disease was stable in ten patients, the curative effect lasted 2.5-15.5 months, and the median progression-free survival was four months.
Patient 1’s CT scans before and after CART-RGFR cell therapy, as well as at 1, 3, 10, and 15 months. The original tumour and retroperitoneal lymph node metastases are shown by red arrows. During chemotherapy pretreatment, ten patients experienced grade 3 to 4 side effects, however they all recovered after active treatment. This is the first time a Chinese-developed CAR-T that targets solid tumours has shown therapeutic success. Congratulations to a patient with severely malignant cholangiocarcinoma who achieved complete remission for the first time.
T-Cell therapy
Melinda Bachini, 41, will never forget the autumn of 2009. She was diagnosed with a rare bile duct cancer on the day of her son’s 14th birthday, and given only a few months to live. She was hesitant and straining. Fortunately, fortune was on her side, and she is now a 13-year survivor of advanced bile duct cancer! Tumor-infiltrating lymphocyte (TIL) therapy has helped Melinda go from having only a few months to live to becoming a super-survivor.
What played a huge role in Melinda was the second infusion of 127 billion immune cells. At first, her tumor volume shrank by 60%. After the trial, she was treated with the immune checkpoint inhibitor pembrolizumab, which successfully shrunk the tumor. Any part of the body is now guaranteed to be cancer-free.
Cancers of the digestive system make up a significant fraction of all human malignancies, accounting for over half of all tumours in my nation. As a result, finding appropriate treatment strategies for digestive tract malignancies is critical. GPC3, CT041, CEA, CD133, and other targets targeted by CAR-T cells could be used to treat liver, gastric, pancreatic, and other digestive system malignancies. Furthermore, major T cell therapies have started a vigorous attack on the three most intractable cancer kings, with the blessing of TCR-T therapy, TILs therapy, and CTL therapy! In conclusion, I believe that as T cell treatment advances, there will undoubtedly be safer and more effective cellular immunotherapy in the near future to successfully treat digestive system malignancies, particularly the three most refractory cancers, considerably extending patient survival.

