Pancreatic cancer
Pancreatic cancer, also referred to as the “king of cancers,” is a highly deadly malignant tumor, with a survival rate of fewer than 10% over a 5-year period. As a result of inadequate screening methods, the majority of individuals with PC typically exhibit advanced or metastatic disease upon diagnosis. The effectiveness of traditional treatment for PC, which includes chemotherapy, radiation, and immune checkpoint inhibitors, is often inadequate.
Furthermore, pancreatic cancer is highly susceptible to relapse and metastasis, even in those who have undergone surgical intervention. As a result, the prognosis for pancreatic cancer is exceedingly grim. Hence, there is an urgent requirement for novel and efficacious therapeutic approaches for PC.
Chimeric antigen receptor T (CAR-T) cell treatment is a highly promising form of adoptive T cell therapy, particularly in the field of cancer immunotherapy. Recently, it has demonstrated significant accomplishments in the treatment of blood cancers, such as relapsed/refractory non-Hodgkin lymphoma, B-cell acute lymphocytic leukemia, and multiple myeloma.
Multiple clinical trials have shown a cure rate of over 80%. Given the remarkable effectiveness of CAR-T cell therapy in treating blood cancer, researchers are currently investigating its potential application in treating solid tumors. Claudin18.2, a variant of claudin18, belongs to a group of proteins called tight junction proteins. It is only found in specialized epithelial cells of the stomach mucosa in normal tissues.
But during the growth of cancer, cells lose their polarity, which makes claudin 18.2 epitopes appear. These epitopes are linked to stomach cancer, cancer of the gastroesophageal junction, and PC. Therefore, claudin 18.2 has a significant level of expression in these cancerous tumors. Furthermore, claudin 18.2 is present in distant tumor metastasis and has a role in the growth, specialization, and movement of cancer cells.
A very important finding is that the claudin 18.2 antigen is found in more than half of all PC cases, and it is highly expressed in the vast majority of these cases. Thus, claudin 18.2 presents itself as a suitable candidate for CAR-T cell therapy in cases of metastatic or advanced PC (8–10). In this report, we describe a case where advanced pancreatic cancer was completely eliminated with the use of claudin 18.2-targeted CAR-T cell treatment, resulting in remission.
CASE
On June 11, 2021, a 72-year-old man had a pancreaticoduodenectomy at Queen Mary Hospital in Hong Kong. This procedure was done because he had a space-occupying lesion in his bile duct and increased levels of carbohydrate antigen 19-9 (CA19-9). The results of the postoperative pathology revealed the presence of pancreatic ductal adenocarcinoma, specifically at stage III (pT2N2M0). Subsequently, he underwent 8 cycles of the gemcitabine plus capecitabine combination as postoperative chemotherapy, which continued until February 2022.
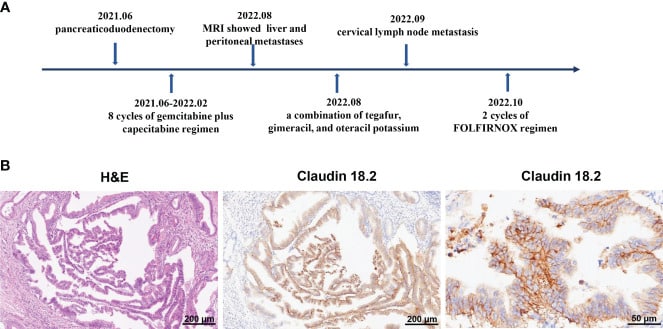
Despite the absence of tumor recurrence on CT scans, the blood levels of CA19-9 steadily increased and exceeded 100 u/mL after adjuvant chemotherapy. On August 5, 2022, magnetic resonance imaging (MRI) revealed the presence of liver metastasis and peritoneal metastasis, indicating the advancement of the disease. Consequently, the chemotherapy treatment was modified to include a mixture of tegafur, gimeracil, and oteracil potassium.
Regrettably, on September 20, 2022, the patient exhibited enlarged left paratracheal lymph nodes, indicating the metastasis of PC cells to the cervical lymph nodes. Subsequently, the patient was transferred to our department to receive additional therapy for metastatic PC. Specifically, the patient underwent two cycles of the FOLFIRINOX regimen (comprising fluorouracil, leucovorin, irinotecan, and oxaliplatin) starting on October 1, 2022. While undergoing FOLFIRINOX chemotherapy, the patient had symptoms such as fatigue, nausea, vomiting, weight loss, neutropenia, and anemia.
The patient declined to continue the FOLFIRINOX regimen due to its low tolerability. Considering the reappearance of the tumor and its spread to other parts of the body despite multiple treatment attempts, the patient and his family expressed a strong desire to be involved in clinical studies involving CAR-T cell therapy. So, immunochemistry was used to find claudin18.2 in tumor tissues.
It showed a significant increase in claudin18.2 expression, with a positive tumor cell rate of over 70%. Consequently, the patient was included in clinical trials investigating the efficacy of claudin18.2-targeted CAR-T cell treatment (NCT05620732).
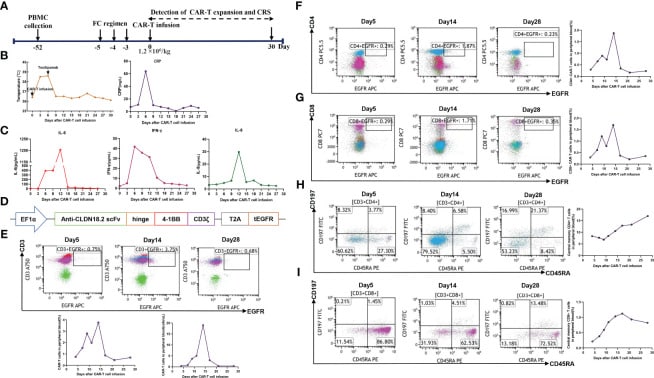
Autologous T cells were harvested from the patient’s peripheral blood on September 30, 2022, for the purpose of producing CAR-T cells that target claudin18.2. These T cells were subsequently modified genetically to express a chimeric antigen receptor (CAR) that includes a humanized anti-claudin18.2 single-chain variable fragment (scFv). Subsequently, the patient had lymphodepleting chemotherapy using the FC regimen (fludarabine 50 mg/m2 from day -5 to day -3, cyclophosphamide 400 mg/m2 from day -5 to day -3) on November 16, 2022.
During the lymphodepletion preconditioning phase, the patient had symptoms such as fever, chills, and upper abdomen pain. However, no abnormalities were seen in the blood culture and hepatic function tests, and there were no indications of acute pancreatitis. Following the administration of empirical anti-infective medication and supportive care, the patient’s clinical status showed improvement.
Due to the patient’s old age and low tolerance, they received a single infusion of claudin 18.2-targeted CAR-T cells at a dose of 1.2 × 106 cells/kg on November 21, 2022. The patient developed a fever three days after receiving CAR-T cell infusion (Figure 2B), along with symptoms of exhaustion, intermittent upper abdomen pain, and nausea. The serum levels of interleukin-6 (IL-6) were markedly increased and reached 389.6 pg/mL on day 6, as shown in Figure 2C.
This finding indicates the presence of grade 2 cytokine release syndrome (CRS). Thus, tocilizumab was employed to mitigate inflammatory reactions. On the ninth day, the patient experienced diarrhea, which was managed with symptomatic treatment including the use of gentamicin and antidiarrheal medications, as well as maintaining proper hydration and electrolyte levels.
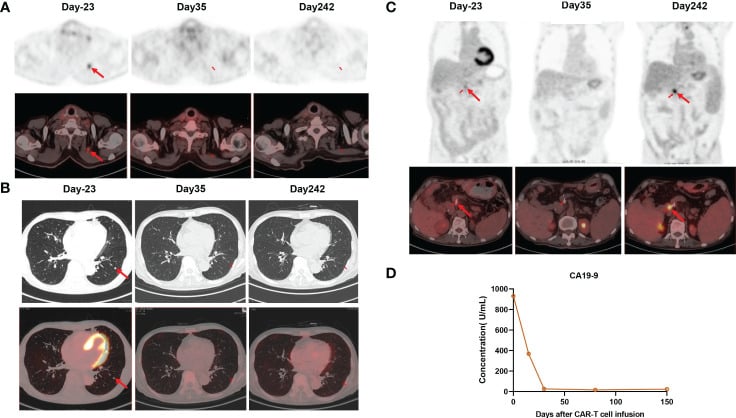
The expansion of CAR-T cells reached its highest levels of 3.75% with 19 cells per microliter in the peripheral blood on day 14. Both CD4 CAR-T cells and CD8 CAR-T cells also achieved their peak blood levels on day 14. Surprisingly, the level of the tumor marker CA19-9 dropped from 1100 U/mL to 25.85 U/mL, and within a month of the CAR-T cells being infused, it returned to normal. Additionally, the PET-CT scan revealed the complete disappearance of tumor lesions, including cervical lymph node metastasis and lung metastasis.
Following a thorough clinical evaluation, the patient experienced a total remission one month after receiving CAR-T cell treatment specifically targeting claudin 18.2. To add to that, the number of central memory CD4+ T cells increased significantly one month after claudin 18.2-targeted CAR-T cell treatment.
However, the percentage of central memory CD8+ T cells reached its highest point on day 17 and thereafter decreased. CA19-9 levels remained within the normal range for 5 months following CAR-T cell therapy, as shown in Figure 3D. Additionally, a PET-CT scan conducted 8 months after CAR-T cell therapy showed no signs of the neck and lung lesions recurring. Regrettably, the PET-CT scan revealed the reappearance of the tumor in its original location 8 months after the infusion of CAR-T cells.
Additionally, the biopsy of the tumor tissues indicated the absence of claudin 18.2. The gastroscopy and biopsy findings showed widespread swelling, increased blood flow, and loss of surface tissue in the stomach, along with a significant presence of lymphocytes infiltrating the area. These changes occurred 2 and 4 months after receiving claudin18.2-targeted CAR-T cell therapy.
However, the use of proton pump inhibitors, prokinetic drugs, and gastric mucosal protectants partially improved these symptoms. Furthermore, a nasogastric tube was employed to provide nutritional assistance, and the patient has since resumed normal meals.
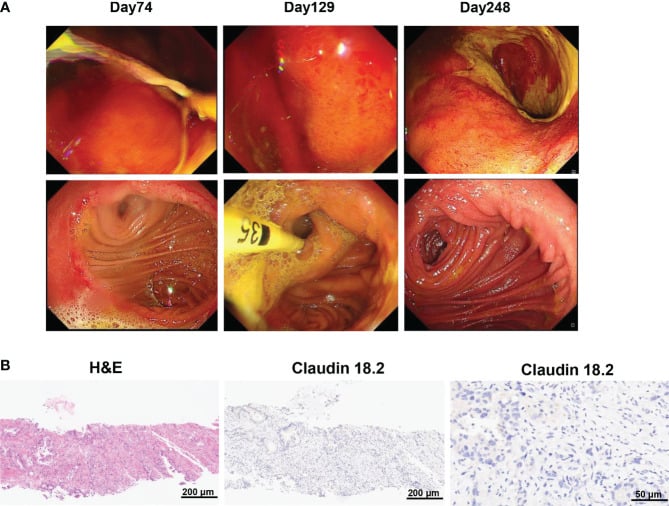
Histopath review
The tumor tissues were preserved in a 4% paraformaldehyde solution for 24 hours in order to prepare them for histological evaluation. Next, the tissues that were embedded in paraffin were sliced into thin sections measuring 4 μm in thickness. These sections were then stained using hematoxylin and eosin (H&E) to visualize the tissue structure.
In order to determine the extent of claudin18.2 expression, the paraffin sections were treated with xylene to remove the paraffin and then gradually rehydrated using different concentrations of alcohol. Following antigen repair using the citrate acid repair solution, the endogenous peroxidase enzyme was deactivated using a 3% H2O2 solution.
Following the application of a 5% BSA blocking solution, the slides were exposed to an anti-claudin 18.2 primary antibody (Rabbit, 1:500, abcam) and a biotinylated secondary antibody (goat, 1:500, Yeasen). Ultimately, the slides were immersed in a DAB chromogenic solution for a duration of 10 minutes and thereafter stained with hematoxylin. The sections were observed using an optical microscope from Carl Zeiss, Germany.
The overall count of PC cells and the count of PC cells that were positive for claudin18.2 were determined. Subsequently, the claudin18.2-positive cell rate was determined by calculating the percentage of claudin18.2-positive cells, which is equal to the number of claudin18.2-positive PC cells divided by the total number of PC cells, multiplied by 100%.
CAR T manufacturing
We got peripheral blood mononuclear cells (PBMCs) through apheresis and then used Ficoll density gradient centrifugation to separate and clean them. Next, T cells were separated and stimulated using CD3/28 Dynabeads. After being activated for a duration of 2 days, the lentiviral vector containing the claudin18.2 CAR gene was used to introduce the genetic material into the T cells. The CAR structure comprises a humanized anti-claudin18.2 single-chain variable fragment (scfv), a CD8α hinge region, a 4-1BB co-stimulatory domain, and a CD3ζ signaling domain.
A nonfunctional epidermal growth factor receptor (tEGFR) was appended to the end of the CAR structure using a T2A linker. The tEGFR served as an indicator for transduction and a safety mechanism for the elimination of cells by the application of anti-EGFR antibodies. The CAR-T cells were cultured in X-vivo media supplemented with IL-7 at a concentration of 10ng/ml, IL-15 at a concentration of 5ng/ml, and IL-21 at a concentration of 30ng/ml until the desired cell amount was achieved.
Flow Cytometry
To find out how the CAR-T cells that were specifically targeting claudin 18.2 grew, 8 mL of peripheral blood were taken at different times using EDTA tubes. Following the destruction of red blood cells, the remaining cells in each tube were treated with anti-human antibodies, specifically EGFR-APC (Biolegend, 52906), CD3- APC/Cyanine7 (Biolegend, 300426), CD4-PerCP/Cyanine5.5 (Biolegend, 300530), CD45RA-FITC (Biolegend, 304106), CD197-PE (Biolegend, 353204), and CD8- PE/Cyanine7 (Biolegend, 344712) for the purpose of flow cytometry analysis.
The presence of these CAR-T cells, which are directed toward claudin 18.2, can be directly identified using CD3-APC/Cyanine7 and tEGFR-APC antibodies. This is possible because tEGFR has been shortened and combined with claudin 18.2-targeted CAR.
Conclusion
To summarize, this study documented a case where advanced PC achieved complete remission following claudin18.2-targeted CAR-T cell therapy. The treatment was accompanied by manageable adverse effects, suggesting that claudin18.2-targeted CAR-T cell therapy could be a highly effective therapeutic approach for advanced PC. CAR T-Cell therapy in China has developed very rapidly in the last few years. The cost of CAR T cell therapy in China is between 50-60,000 USD. The best hospitals for CAR T cell therapy in China are conducting clinical trials in this area.
Given the occurrence of relapse without the presence of antigens 8 months after treatment with claudin18.2-targeted CAR-T cell therapy, it is crucial to discover new surface antigens that are unique to tumors for the treatment of advanced or metastatic PC. Furthermore, current research on the effectiveness and safety of claudin18.2-targeted CAR-T cell treatment in pancreatic cancer is still in its early phases. It is necessary to validate these findings through future, extensive clinical trials.