Latest news in cancer
An Informative Guide On The Signs And Symptoms Of Multiple Myeloma
Read our guide to learn about the silent indicators of multiple myeloma. Learn to spot them early, so you can make smart choices for your health. Don’t...
A Closer Look At The Various Stages Of Multiple Myeloma
Learn about the different stages and types of multiple myeloma. Find hope and support with information on treatments and coping strategies. This simple guide...
How Does Diagnostic Imaging Save Lives in Multiple Myeloma Battles?
Curious about how a medical technique is saving the lives of multiple myeloma patients? Read our blog to learn more about this life-saving technique!...
The Key To CAR-T Success Lies In Patient Selection: Are You The Ideal One?
Discover the magic of CAR-T treatment! Read our blog on patient selection for CAR T therapy. Are you the ideal candidate for this innovative cancer treatment?...
Immunotherapy Can Help You Win the Battle Against Multiple Myeloma!
Discover how immunotherapy can be your true friend in beating myeloma! Our blog provides simple insights into the power of immunotherapy for multiple myeloma....
How Stem Cell Transplant Is Reshaping The Future Of Multiple Myeloma Treatment?
Stem cell transplantation is an effective treatment for multiple myeloma, a type of blood cancer. This procedure has shown promising results in improving the...
CAR T Cells Are Reshaping the Future of Cancer Treatment!
The revolutionary CAR T Cell Therapy is changing the scenario of how we deal with cancer, making it more personal and powerful. This latest therapy uses a...
The Role Of Immunotherapy In Lymphoma Treatment
If you’re reading this, you or maybe one of your loved ones is on a journey that no one ever plans to take—the path of facing cancer. We understand...
Ivosidenib is approved by FDA for myelodysplastic syndromes
The Food and Drug Administration approved ivosidenib (Tibsovo, Servier Pharmaceuticals LLC) for adult patients with relapsed or refractory myelodysplastic...
Neoadjuvant/ adjuvant pembrolizumab is approved by the FDA for resectable non-small cell lung cancer
The Food and Drug Administration approved pembrolizumab (Keytruda, Merck) with platinum-containing chemotherapy as neoadjuvant treatment, and with continuation...
Nivolumab is approved by the FDA for adjuvant treatment of Stage IIB/C melanoma
The Food and Drug Administration approved nivolumab (Opdivo, Bristol-Myers Squibb Company) for the adjuvant treatment of completely resected Stage IIB/C...
Encorafenib with binimetinib is approved by the FDA for treatment of metastatic non-small cell lung cancer with a BRAF V600E mutation
The Food and Drug Administration approved encorafenib (Braftovi, Array BioPharma Inc., a wholly owned subsidiary of Pfizer) with binimetinib (Mektovi, Array...
Bosutinib is approved by the FDA for pediatric patients with chronic myelogenous leukemia
The Food and Drug Administration approved bosutinib (Bosulif, Pfizer) for pediatric patients 1 year of age and older with chronic phase (CP) Ph+ chronic...
New and updated indications are approved by FDA for temozolomide under Project Renewal
The Food and Drug Administration (FDA) approved updated labeling for temozolomide (Temodar, Merck) under Project Renewal, an Oncology Center of Excellence...
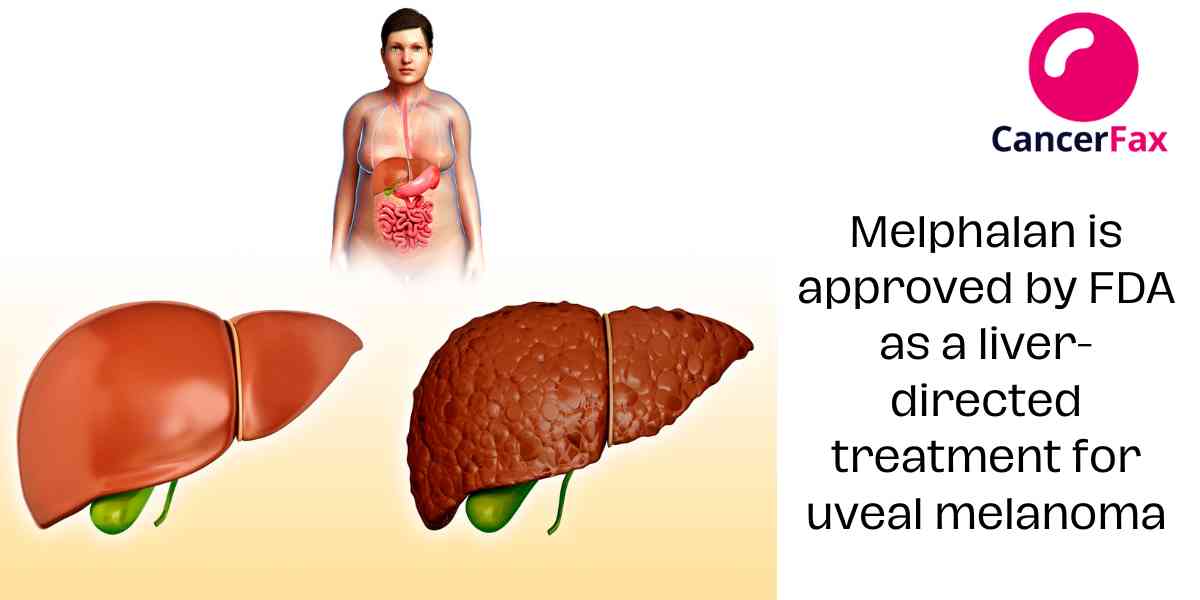
Melphalan is approved by FDA as a liver-directed treatment for uveal melanoma
HEPZATO KIT (melphalan for Injection/Hepatic Delivery System, Delcath Systems, Inc.) was approved by the Food and Drug Administration as a liver-directed...
Elranatamab-bcmm receives accelerated approval by FDA for multiple myeloma
Elranatamab-bcmm (Elrexfio, Pfizer, Inc.) is a bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager that was given accelerated approval by...
CAR T Cell Therapy: How Does It Work?
Discover the science behind CAR T Cell therapy treatment in India! Explore how this revolutionary treatment transforms your immune cells into cancer fighters....
Is CAR T-Cell Therapy Available In India?
Have you ever wondered if there is a powerful way to fight cancer? Now just imagine if one day you found a ray of hope in your fight against cancer, a...
Niraparib and abiraterone acetate plus prednisone is approved by FDA for BRCA-mutated metastatic castration-resistant prostate cancer
The Food and Drug Administration approved the fixed dose combination of niraparib and abiraterone acetate (Akeega, Janssen Biotech, Inc.), with prednisone, for...
Talquetamab-tgvs has received accelerated approval for relapsed or refractory multiple myeloma
The Food and Drug Administration granted accelerated approval to talquetamab-tgvs (Talvey, Janssen Biotech, Inc.) for adults with relapsed or refractory...
Pralsetinib is approved by the FDA for non-small cell lung cancer with RET gene fusions
August 2023: Pralsetinib (Gavreto, Genentech, Inc.) was given regular approval by the Food and Drug Administration for adult patients with metastatic RET...
Trifluridine and tipiracil with bevacizumab is approved by FDA for previously treated metastatic colorectal cancer
The Food and Drug Administration approved trifluridine and tipiracil (LONSURF, Taiho Oncology, Inc.) with bevacizumab, for metastatic colorectal cancer (mCRC)...
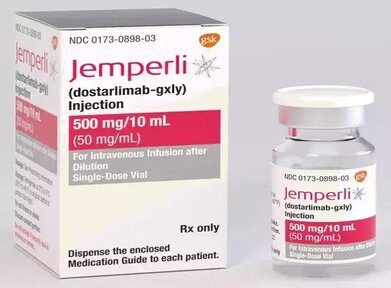
Dostarlimab-gxly with chemotherapy is approved by the FDA for endometrial cancer
The Food and Drug Administration approved dostarlimab-gxly (Jemperli, GlaxoSmithKline) with carboplatin and paclitaxel, followed by single-agent...
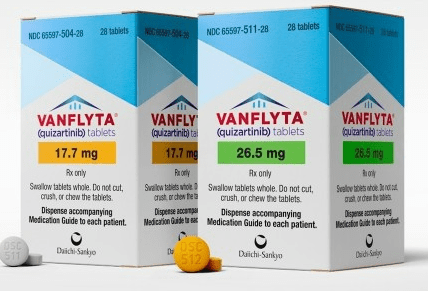
Quizartinib is approved by the FDA for newly diagnosed acute myeloid leukemia
The Food and Drug Administration approved quizartinib (Vanflyta, Daiichi Sankyo, Inc.) with standard cytarabine and anthracycline induction and cytarabine...
A trial shows that blinatumomab could be used in more ways to treat ALL
BLINCYTO® (blinatumomab) is a prescription medicine used to treat B-cell precursor acute lymphoblastic leukemia (ALL) in patients who still have detectable...
Development and future potential of the BiTE (bispecific T-cell engager) platform
Immuno-oncology is a way to treat cancer by using the body's immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform...
Rare autoimmune disorder myasthenia gravis treated by CAR-T therapy
The modified CAR-T treatment, which stands for chimeric antigen receptor T-cell, was used by scientists. It could reduce myasthenia gravis symptoms for a...
Talazoparib with enzalutamide is approved by FDA for HRR gene-mutated metastatic castration-resistant prostate cancer
The Food and Drug Administration approved talazoparib (Talzenna, Pfizer, Inc.) with enzalutamide for homologous recombination repair (HRR) gene-mutated...
Glofitamab-gxbm is approved by FDA for selected relapsed or refractory large B-cell lymphomas
July 2023: The Food and Drug Administration gave accelerated approval to glofitamab-gxbm (Columvi, Genentech, Inc.) for relapsed or refractory diffuse large...
Olaparib with abiraterone and prednisone (or prednisolone) is approved by FDA for BRCA-mutated metastatic castration-resistant prostate cancer
July 2023: The Food and Drug Administration (FDA) approved olaparib (Lynparza, AstraZeneca Pharmaceuticals LP) with abiraterone and prednisone (or...
Epcoritamab-bysp is approved by FDA for relapsed or refractory diffuse large B-cell lymphoma and high-grade B-cell lymphoma
July 2023: The Food and Drug Administration gave accelerated approval to epcoritamab-bysp (Epkinly, Genmab US, Inc.) for relapsed or refractory diffuse large...
Companies in Korea takes a step closer in developing home grown CAR T-Cell therapy
Due to high costs, treatments developed by multinational pharmaceutical corporations are difficult for Korean patients to access. As a result, Korean...
Polatuzumab vedotin-piiq is approved by USFDA for previously untreated diffuse large B-cell lymphoma, not otherwise specified, and high-grade B-cell lymphoma
For adult patients with high-grade B-cell lymphoma (HGBL), not otherwise specified (NOS), or diffuse large B-cell lymphoma (DLBCL) who have not previously...
Omidubicel is approved by USFDA to reduce time to neutrophil recovery and infection in patients with hematologic malignancies
Omidubicel-onlv (Omisirge, Gamida Cell Ltd.) was approved by the Food and Drug Administration for use in adult and paediatric patients (12 years of age and...
Enfortumab vedotin-ejfv with pembrolizumab is approved by USFDA for locally advanced or metastatic urothelial carcinoma
Enfortumab vedotin-ejfv (Padcev, Astellas Pharma) and pembrolizumab (Keytruda, Merck) have been given accelerated approval by the Food and Drug Administration...
Retifanlimab-dlwr is approved by FDA for metastatic or recurrent locally advanced Merkel cell carcinoma
Retifanlimab-dlwr (Zynyz, Incyte Corporation) received fast approval from the Food and Drug Administration to treat adult patients with metastatic or recurrent...
Need help? Our team is ready to assist you.
We wish a speedy recovery of your dear and near one.