Immuno-oncology is a way to treat cancer by using the body’s immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform that binds a patient’s own T cells to cancer cells. Because BiTE technology is flexible, it is easy to make molecules that attack tumor-specific antigens, which makes immuno-oncotherapy possible. Blinatumomab was the first standard BiTE molecule to be approved. It targets CD19 surface antigens on B cells and is mostly unaffected by genetic changes or escape mechanisms inside cells. More BiTE molecules are being made to treat other blood cancers (like multiple myeloma, acute myeloid leukaemia, and B-cell non-Hodgkin lymphoma) and solid tumours (like prostate cancer, glioblastoma, stomach cancer, and small-cell lung cancer). BiTE molecules that have a longer half-life than the standard ones are also being made. With BiTE technology, advances in immuno-oncology could make it easier to treat both blood and solid tumours and make them more effective when used with other treatments.
What is BiTe therapy?
Immuno-oncology therapies are scientifically proven ways to treat different types of solid and blood cancers. Hematologic cancers are a good fit for treatments that target the immune system because cancerous blood cells move around with immune cells. Several immunotherapy treatments for cancer are in the works.
Monoclonal antibody checkpoint inhibitors that stop the binding of checkpoint proteins (like PD-1 and CTLA-4) are useful against many types of cancer. They work well and are safe for many solid tumours, especially when they target PD-1. Non-small-cell lung, kidney, and bladder cancers have all been treated successfully with these drugs. But many people don’t react to checkpoint inhibitors or get sick again after taking them. Except for non-Hodgkin lymphoma, most results on hematologic cancers have been disappointing, especially for myeloma and leukaemia, where the overall response rate in approved indications ranges from 12.0% to 48.5%.8-15.
Other immuno-oncology treatments, on the other hand, have a higher success rate. Chimeric antigen-receptor (CAR) T-cell therapies change a patient’s T cells to attack a specific cellular antigen, such as CD19 in the treatment of B-cell malignancies and B-cell maturation antigen (BCMA) in the treatment of multiple myeloma (MM). CAR T-cell treatments have shown promise in treating hematologic cancers. They haven’t been as effective in treating solid tumours, but there have been some good results with neuroblastoma, human epidermal growth factor receptor tumours, and non-small-cell lung cancer. The genetic modification and in vitro multiplication of T cells take a long and complicated manufacturing process. This is a downside of this therapy because it makes it harder for patients to get this treatment quickly and in large numbers. The fact that lymphodepletion through chemotherapy preparation must be done first as a requirement for improved effectiveness is also a drawback.
BiTE (bispecific T-cell engager) therapies link a patient’s own T cells to tumor-expressed antigens. This turns on the cytotoxic ability of the patient’s own T cells to kill cancer without changing the T cells’ genes or needing to grow or manipulate them outside of the body. BiTE molecules can be used alone as medicines or with other treatments to make them more effective.
BiTe mechanism of action
BiTE molecules are antibody constructs with two binding domains. One recognises tumor-expressed antigens (such as BCMA, CD19, or -like protein [DLL3]), and the other, CD3, recognises T cells (Fig. 1). Two single-chain variable fragment (scFv) regions from monoclonal antibodies are connected by a flexible peptide linker to make the binding domains. The first scFv binding region can be changed to target any surface antigen, so it can be used right away to treat a wide range of tumours and can be used again later. The second scFv binding region always binds to CD3, which is a part of the T-cell receptor complex that never changes. When a BiTE molecule interacts with both a cytotoxic T cell and a tumour cell, the T cells begin to multiply. This increases the amount of effector cells and makes BiTE therapy more effective. Then, the death of cancer cells is started. BiTE molecules can get any T cells to do this because they don’t need co-stimulation or the usual processes of the major histocompatibility complex.
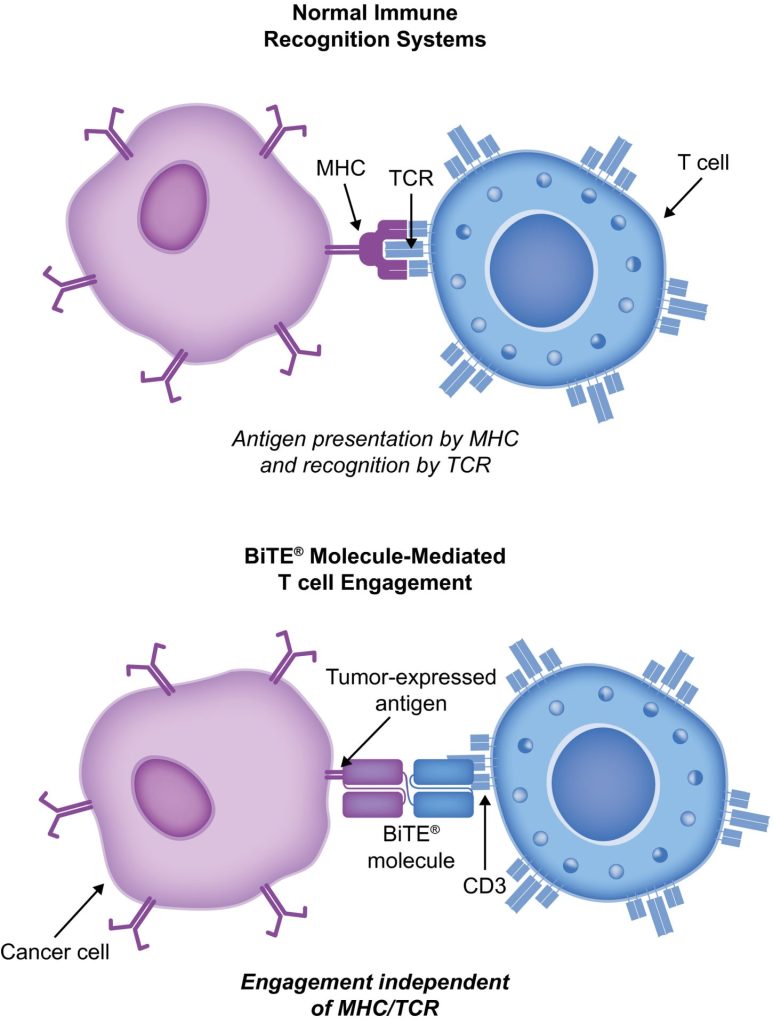
Blinatumomab is the first and only BiTE therapy that has been approved. It targets the CD19 receptor on both normal and cancerous B cells. It is a highly potent molecule with cytotoxic effects seen at low exposures (10–100 pg/mL)26. In its presence, T cells can perform serial-target lysis, quickly binding to and killing many cells. This is how BiTE therapies work, and it can be seen in other BiTE molecules that are still in research. In acute lymphoblastic leukaemia (ALL), blinatumomab has been shown to be effective and safe. In 2014, the US Food and Drug Administration gave it fast approval, and in 2017, it got full approval for relapsed or refractory (R/R) B-cell precursor (BCP) ALL. In 2018, accelerated approval was given to blinatumomab for treating BCP-ALL with minimum residual disease (MRD). This was the first approval for this use. In November 2015, the European Medicines Agency also gave it a green light for BCP-ALL with a Philadelphia chromosome (Ph) that is negative and R/R. Blinatumomab is approved for R/R BCP-ALL in adults and children in 57 countries, including Japan, all countries in the European Union, Canada, and Australia.
Blinatumomab for the treatment of patients with BCP-ALL
Blinatumomab has changed the way BCP-ALL is treated. Compared to standard-of-care (SOC) chemotherapy, it has increased overall survival (OS) and decreased the number of certain side effects (AEs). Several important studies, including randomized controlled trials, showed that blinatumomab is safe and works for BCP-ALL in both adults and children.For CAR T-cell therapy, there are only data from 2 single-arm studies (clinicaltrials.gov IDs NCT01626495 and NCT01029366) in which 25 children (ages 5–22) and 5 adults (ages 26–60) with R/R BCP-ALL and T-cell ALL were treated. But the results are promising (a complete response [CR] in 90%, sustained remission with 6-month event-free survival in 67%, and an overall survival [OS] rate of 78% [median follow-up, 7 months; range, 1–24 months]).
The TOWER study (A Phase 3, Randomized, Open Label Study Investigating the Efficacy of the BiTE Antibody Blinatumomab Versus Standard of Care Chemotherapy in Adult Subjects With Relapsed/Refractory B-Precursor ALL; clinicaltrials.gov identifier NCT02013167) compared the effects of blinatumomab monotherapy against SOC chemotherapy in heavily pretreated adults with Ph-negative, R/R BCP-ALL. Because people were living longer, the study was stopped early. AEs in the blinatumomab group were the same as those seen in earlier studies, and blinatumomab had lower exposure-adjusted AE rates than SOC.34 Blinatumomab also works for people with Ph-positive, R/R BCP-ALL and for children with Ph-negative, R/R BCP-ALL.
30% to 50% of people with BCP-ALL in complete hematologic remission show persistent MRD. In the single-arm, phase 2 BLAST study (A Confirmatory Multicenter, Single-Arm Study to Assess the Efficacy, Safety, and Tolerability of the BiTE Antibody Blinatumomab in Adult Patients With MRD of B-Precursor Acute Lymphoblastic Leukaemia; clinicaltrials.gov identifier NCT01207388), blinatumomab was tested on patients with BCP-ALL in first or later complete After blinatumomab treatment, 78% of patients who were MRD positive became MRD negative. The 5-year OS study showed a median OS of 36.5 months, and more than half of those who had a complete MRD response after the first cycle of blinatumomab were still alive at 5 years, which suggests that the treatment might be able to cure some patients. AEs were seen that were linked to cytokine release syndrome (CRS).31 Other studies, like NCT03023878 and NCT03340766, are still looking at blinatumomab in first-line settings and in combination with other treatments.
CD19-targeted treatments have been linked to failure because of the loss of CD19 antigen after treatment. The failure rates for blinatumomab range from 8% to 35%, and for CAR T-cell therapies, they range from 39% to 65%.36-40 We don’t fully understand what causes therapy to fail, but one possibility is immunoediting, in which antigen loss is caused by a T-cell-dependent process called immunoselection, which lets tumour cells get away.41 Lineage switch and epitope loss under therapy pressure have also been suggested as ways for tumours to escape treatment. However, a recent study on epitope loss found that some CD19 isoforms that help CAR T-cells escape were already present at the time of diagnosis. This suggests that combining treatments might be helpful. Another thing that can cause immunotherapy to fail is called “inhibitory T-cell signalling.” In this case, the blocking programmed death ligand-1 (PD-L1) is interesting because it is more common in B-cell ALL cells from patients who don’t respond to blinatumomab and can make CD3 BiTE molecules less effective.43 By making a CD28/PD-L1 BiTE that triggers the CD28 co-stimulatory signal instead of the inhibitory signaling pathway that is usually seen when a T cell binds to a PD-L1-expressing cancer cell, this inhibition could be turned off.43 Dual-targeted CAR T cells are also being looked into as a way to make up for the loss of tumour antigens. This can be done by modifying each T cell with 2 CAR molecules and 2 different binding domains (dual-signaling CAR) or by putting 2 different binding domains on 1 CAR molecule at the same time (TanCAR).
Adverse events with BiTE and its management
In clinical studies of blinatumomab, the most common AEs are fever, low white blood cell count, and low platelet count. Some of the most important risks are CRS, neurotoxicity, and drug mistakes. Neurotoxicity can also happen with CD19-specific CAR T-cell treatments, but it might not be because of CD19. The results of a phase 1/1b study that is still going on about CD20/CD3 targets showed that CNS AEs of grade 3 or higher were rare (3% of all grade 3 AEs). Most of the time, blinatumomab’s reaction to CRS is mild, but in rare cases, it can be severe and even life-threatening. Inflammatory reactions can be lessened with corticosteroids. To lower the chance of CRS, it is best to give an infusion of prednisone or dexamethasone before the first dose of blinatumomab and to increase the dose slowly. This use of corticosteroids before other BiTE molecules has given a reason to use dexamethasone as a premedication when using other BiTE molecules. However, it is not clear if this effect can be applied to the whole BiTE platform, and other ways to deal with CRS are being looked into. Interleukin 6 is a cytokine that causes CRS and is high in people who have it. Tocilizumab, which blocks the interleukin-6 receptor, has been used to treat CRS that is very bad after CAR T-cell treatment.49 In the hospital, tumour necrosis factor- inhibitors have also been used to treat CRS.


