Immuno-oncology is a way to treat cancer by using the body’s immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform that binds a patient’s own T cells to cancer cells. Because BiTE technology is flexible, it is easy to make molecules that attack tumor-specific antigens, which makes immuno-oncotherapy possible. Blinatumomab was the first standard BiTE molecule to be approved. It targets CD19 surface antigens on B cells and is mostly unaffected by genetic changes or escape mechanisms inside cells. More BiTE molecules are being made to treat other blood cancers (like multiple myeloma, acute myeloid leukaemia, and B-cell limfoma bukan Hodgkin) and solid tumours (like prostate cancer, glioblastoma, stomach cancer, and small-cell lung cancer). BiTE molecules that have a longer half-life than the standard ones are also being made. With BiTE technology, advances in immuno-oncology could make it easier to treat both blood and solid tumours and make them more effective when used with other treatments.
Apakah terapi BiTe?
Immuno-oncology therapies are scientifically proven ways to treat different types of solid and barah darah. Hematologic cancers are a good fit for treatments that target the immune system because cancerous blood cells move around with immune cells. Several imunoterapi rawatan untuk kanser sedang dalam kerja-kerja.
Monoclonal antibody checkpoint inhibitors that stop the binding of checkpoint proteins (like PD-1 and CTLA-4) are useful against many types of cancer. They work well and are safe for many solid tumours, especially when they target PD-1. Non-small-cell lung, kidney, and bladder cancers have all been treated successfully with these drugs. But many people don’t react to checkpoint inhibitors or get sick again after taking them. Except for non-Hodgkin limfoma, most results on hematologic cancers have been disappointing, especially for myeloma and leukaemia, where the overall response rate in approved indications ranges from 12.0% to 48.5%.8-15.
Other immuno-oncology treatments, on the other hand, have a higher success rate. Chimeric antigen-receptor (CAR) T-cell therapies change a patient’s T cells to attack a specific cellular antigen, such as CD19 in the treatment of B-cell malignancies and B-cell maturation antigen (BCMA) in the treatment of pelbagai myeloma (MM). CAR T-cell treatments have shown promise in treating hematologic cancers. They haven’t been as effective in treating solid tumours, but there have been some good results with neuroblastoma, human epidermal growth factor receptor tumours, and non-small-cell lung cancer. The genetic modification and in vitro multiplication of T cells take a long and complicated manufacturing process. This is a downside of this therapy because it makes it harder for patients to get this treatment quickly and in large numbers. The fact that lymphodepletion through chemotherapy preparation must be done first as a requirement for improved effectiveness is also a drawback.
Terapi BiTE (bispecific T-cell engager) menghubungkan sel T pesakit sendiri kepada antigen yang diungkapkan tumor. Ini menghidupkan keupayaan sitotoksik sel T pesakit sendiri untuk membunuh kanser tanpa mengubah gen sel T atau perlu membesar atau memanipulasinya di luar badan. Molekul BiTE boleh digunakan secara bersendirian sebagai ubat atau dengan rawatan lain untuk menjadikannya lebih berkesan.
Mekanisme tindakan BiTe
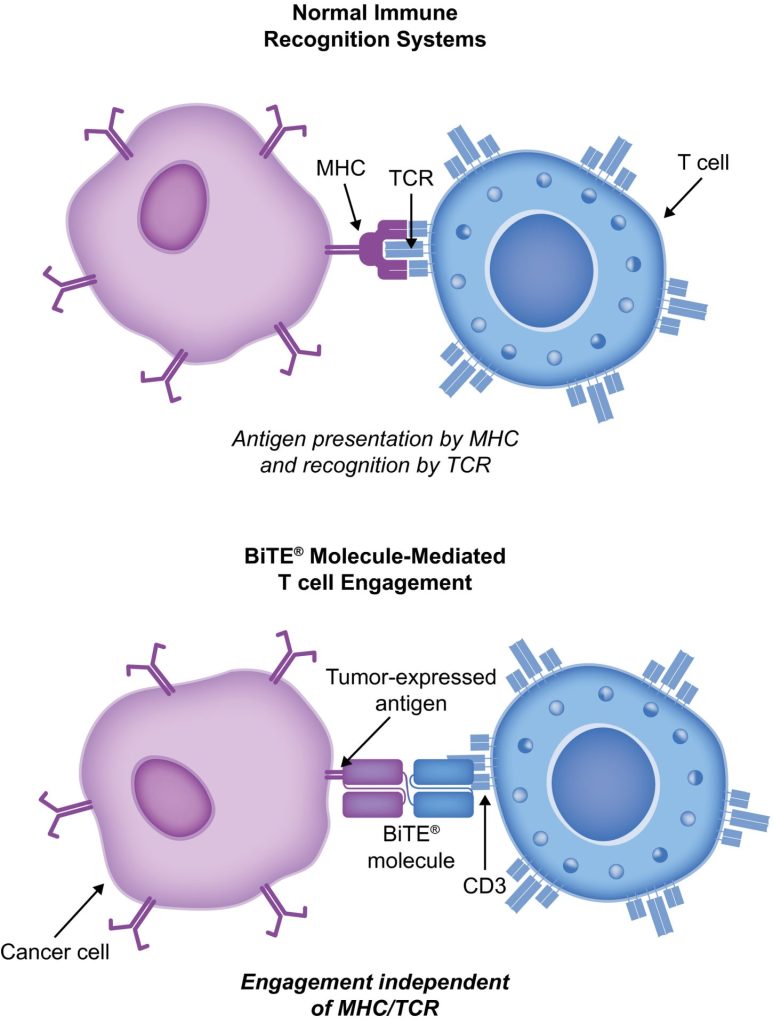
BiTE molecules are antibody constructs with two binding domains. One recognises tumor-expressed antigens (such as BCMA, CD19, or -like protein [DLL3]), and the other, CD3, recognises T cells (Fig. 1). Two single-chain variable fragment (scFv) regions from monoclonal antibodies are connected by a flexible peptide linker to make the binding domains. The first scFv binding region can be changed to target any surface antigen, so it can be used right away to treat a wide range of tumours and can be used again later. The second scFv binding region always binds to CD3, which is a part of the T-cell receptor complex that never changes. When a BiTE molecule interacts with both a cytotoxic T cell and a tumour cell, the T cells begin to multiply. This increases the amount of effector cells and makes BiTE therapy more effective. Then, the death of cancer cells is started. BiTE molecules can get any T cells to do this because they don’t need co-stimulation or the usual processes of the major histocompatibility complex.
Blinatumomab is the first and only BiTE therapy that has been approved. It targets the CD19 receptor on both normal and cancerous B cells. It is a highly potent molecule with cytotoxic effects seen at low exposures (10–100 pg/mL)26. In its presence, T cells can perform serial-target lysis, quickly binding to and killing many cells. This is how BiTE therapies work, and it can be seen in other BiTE molecules that are still in research. In leukemia limfoblastik akut (SEMUA), blinatumomab has been shown to be effective and safe. In 2014, the US Food and Drug Administration gave it fast approval, and in 2017, it got full approval for relapsed or refractory (R/R) B-cell precursor (BCP) ALL. In 2018, accelerated approval was given to blinatumomab for treating BCP-ALL with minimum residual disease (MRD). This was the first approval for this use. In November 2015, the European Medicines Agency also gave it a green light for BCP-ALL with a Philadelphia chromosome (Ph) that is negative and R/R. Blinatumomab is approved for R/R BCP-ALL in adults and children in 57 countries, including Japan, all countries in the European Union, Canada, and Australia.
Blinatumomab untuk rawatan pesakit dengan BCP-ALL
Blinatumomab telah mengubah cara BCP-ALL dirawat. Berbanding dengan kemoterapi standard-of-care (SOC), ia telah meningkatkan kemandirian keseluruhan (OS) dan mengurangkan bilangan kesan sampingan (AEs) tertentu. Beberapa kajian penting, termasuk ujian terkawal rawak, menunjukkan bahawa blinatumomab adalah selamat dan berfungsi untuk BCP-ALL dalam kedua-dua orang dewasa dan kanak-kanak. Terapi sel T KERETA, hanya terdapat data daripada 2 kajian lengan tunggal (clinicaltrials.gov IDs NCT01626495 dan NCT01029366) di mana 25 kanak-kanak (umur 5–22) dan 5 orang dewasa (umur 26–60) dengan R/R BCP-ALL dan T-cell ALL telah dirawat. Tetapi keputusannya menjanjikan (tindak balas lengkap [CR] dalam 90%, pengampunan yang berterusan dengan kelangsungan hidup tanpa peristiwa selama 6 bulan dalam 67%, dan kadar kelangsungan hidup keseluruhan [OS] sebanyak 78% [tindakan median, 7 bulan; julat, 1-24 bulan]).
Kajian TOWER (A Fasa 3, Rawak, Kajian Label Terbuka Menyiasat Keberkesanan Antibodi BiTE Blinatumomab Berbanding Kemoterapi Standard Penjagaan dalam Subjek Dewasa Dengan Relaps/Refractory B-Precursor ALL; clinicaltrials.gov pengecam NCT02013167) berbanding kesan Blinatumomab-Phoctreatively kemoterapi dewasa dengan kemoterapi Blinatumomab, R. CP-SEMUA. Kerana orang hidup lebih lama, kajian itu dihentikan lebih awal. AE dalam kumpulan blinatumomab adalah sama seperti yang dilihat dalam kajian terdahulu, dan blinatumomab mempunyai kadar AE terlaras pendedahan yang lebih rendah daripada SOC.34 Blinatumomab juga berfungsi untuk orang yang mempunyai Ph-positif, R/R BCP-ALL dan untuk kanak-kanak dengan Ph-negatif, R/R BCP-ALL.
30% to 50% of people with BCP-ALL in complete hematologic remission show persistent MRD. In the single-arm, phase 2 BLAST study (A Confirmatory Multicenter, Single-Arm Study to Assess the Efficacy, Safety, and Tolerability of the BiTE Antibody Blinatumomab in Adult Patients With MRD of B-Precursor Acute Lymphoblastic Leukaemia; clinicaltrials.gov identifier NCT01207388), blinatumomab was tested on patients with BCP-ALL in first or later complete After blinatumomab treatment, 78% of patients who were MRD positive became MRD negative. The 5-year OS study showed a median OS of 36.5 months, and more than half of those who had a complete MRD response after the first cycle of blinatumomab were still alive at 5 years, which suggests that the treatment might be able to cure some patients. AEs were seen that were linked to sindrom pelepasan sitokin (CRS).31 Other studies, like NCT03023878 and NCT03340766, are still looking at blinatumomab in first-line settings and in combination with other treatments.
CD19-targeted treatments have been linked to failure because of the loss of CD19 antigen after treatment. The failure rates for blinatumomab range from 8% to 35%, and for Terapi sel T CAR, they range from 39% to 65%.36-40 We don’t fully understand what causes therapy to fail, but one possibility is immunoediting, in which antigen loss is caused by a T-cell-dependent process called immunoselection, which lets tumour cells get away.41 Lineage switch and epitope loss under therapy pressure have also been suggested as ways for tumours to escape treatment. However, a recent study on epitope loss found that some CD19 isoforms that help CAR T-cells escape were already present at the time of diagnosis. This suggests that combining treatments might be helpful. Another thing that can cause immunotherapy to fail is called “inhibitory T-cell signalling.” In this case, the blocking programmed death ligand-1 (PD-L1) is interesting because it is more common in B-cell ALL cells from patients who don’t respond to blinatumomab and can make CD3 BiTE molecules less effective.43 By making a CD28/PD-L1 BiTE that triggers the CD28 co-stimulatory signal instead of the inhibitory signaling pathway that is usually seen when a T cell binds to a PD-L1-expressing cancer cell, this inhibition could be turned off.43 Dual-targeted CAR T cells are also being looked into as a way to make up for the loss of tumour antigens. This can be done by modifying each T cell with 2 CAR molecules and 2 different binding domains (dual-signaling CAR) or by putting 2 different binding domains on 1 CAR molecule at the same time (TanCAR).
Kejadian buruk dengan BiTE dan pengurusannya
Dalam kajian klinikal blinatumomab, AE yang paling biasa ialah demam, kiraan sel darah putih yang rendah, dan kiraan platelet yang rendah. Beberapa risiko yang paling penting ialah CRS, neurotoksisiti, dan kesilapan dadah. Neurotoksisiti juga boleh berlaku dengan rawatan sel T CAR khusus CD19, tetapi ia mungkin bukan disebabkan oleh CD19. Keputusan kajian fasa 1/1b yang masih berjalan kira-kira sasaran CD20/CD3 menunjukkan bahawa AE CNS gred 3 atau lebih tinggi adalah jarang berlaku (3% daripada semua AE gred 3). Selalunya, tindak balas blinatumomab terhadap CRS adalah ringan, tetapi dalam kes yang jarang berlaku, ia boleh menjadi teruk dan bahkan mengancam nyawa. Reaksi keradangan boleh dikurangkan dengan kortikosteroid. Untuk mengurangkan kemungkinan CRS, sebaiknya berikan infusi prednison atau dexamethasone sebelum dos pertama blinatumomab dan tingkatkan dos secara perlahan. Penggunaan kortikosteroid ini sebelum molekul BiTE lain telah memberi alasan untuk menggunakan deksametason sebagai premedikasi apabila menggunakan molekul BiTE lain. Walau bagaimanapun, tidak jelas sama ada kesan ini boleh digunakan pada keseluruhan platform BiTE, dan cara lain untuk menangani CRS sedang diteliti. Interleukin 6 ialah sitokin yang menyebabkan CRS dan tinggi pada orang yang menghidapnya. Tocilizumab, yang menyekat reseptor interleukin-6, telah digunakan untuk merawat CRS yang sangat teruk selepas rawatan sel T CAR.49 Di hospital, perencat faktor nekrosis tumor juga telah digunakan untuk merawat CRS.


