Janssen Biotech, MonumenTAL-1, multiple myeloma, Talquetamab-tgvs, Talvey
Talquetamab-tgvs has received accelerated approval for relapsed or refractory multiple myeloma
August 2023: Talquetamab-tgvs (Talvey, Janssen Biotech, Inc.) has been given accelerated approval by the Food and Drug Administration for the treatment of adults with relapsed or refractory multiple myeloma who have undergone at ..

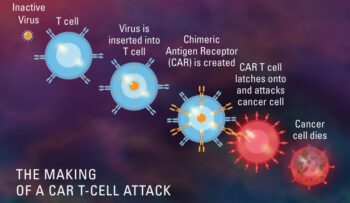
CAR T Cell therapy trials, multiple myeloma trials
Clinical trial on CAR-T Cell therapy for patients with BCMA/TACI-positive relapsed and/or refractory multiple myeloma
Brief Summary: A study of APRIL CAR-T cells therapy for patients with BCMA/TACI positive relapsed and/or refractory multiple myeloma Detailed Description: This is a single arm, open-label, si..

Arcellx, CAR T Therapy, CART DDBCMA, chimeric antigen receptor, Kite Pharma, multiple myeloma
KITE AND ARCELLX CLOSE AGREEMENT TO CO-DEVELOP AND CO-COMMERCIALIZE LATE-STAGE CLINICAL CART-DDBCMA IN MULTIPLE MYELOMA
SANTA MONICA, Calif. & REDWOOD CITY, Calif.--(BUSINESS WIRE)-- Kite, a Gilead Company (NASDAQ: GILD), and Arcellx, Inc. (NASDAQ: ACLX), today announced the closing of the companies’ previously announced global strategic..

Beijing, CARTITUDE-4, CARVYKTI, China, ciltacabtagene autoleucel, Clinical trials, Legend biotech, refractory multiple myeloma, Shanghai
Legend Biotech Announces Phase 3 CARTITUDE-4 Study of CARVYKTI®(ciltacabtagene autoleucel) Has Met Its Primary Endpoint in the Treatment of Patients with Relapsed and Refractory Multiple Myeloma
JANUARY 27, 2023—Legend Biotech Corporation (NASDAQ: LEGN) (Legend Biotech), a global biotechnology company developing, manufacturing and commercializing novel therapies to treat life-threatening diseases, announced today that ..
anti-BCMA, CAR - T Cell therapy, CT103A, FDA, IASO Biotherapeutics, Innovent Biologics, orphan drug designation
CT103A, CAR T-cell Therapy, has been designated as an orphan drug by the FDA
Feb 2023: The FDA has granted orphan drug status to CT103A, an experimental CAR T-cell therapy being developed by IASO Biotherapeutics and Innovent Biologics to treat relapsed or refractory multiple myeloma. Orphan drug design..
CAR - T Cell therapy, China, IASO Biotherapeutics, multiple myeloma
CAR T-cell treatment from IASO Biotherapeutics receives new FDA approval
Feb 2023: IASO Biotherapeutics' investigational CAR T-cell therapy for relapsed or refractory multiple myeloma (RRMM), CT103A, has received fast track and regenerative medicine advanced therapy designations from the U.S. Food and..
Janssen Biotech, MajesTEC-1, NCT03145181, teclistamab-cqyv, Tecvayli
Teclistamab-cqyv is approved by FDA for relapsed or refractory multiple myeloma
November 2022: The first bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager, teclistamab-cqyv (Tecvayli, Janssen Biotech, Inc.), was given accelerated approval by the Food and Drug Administration for adult pat..
BCMA, CARVYKTI, ciltacabtagene autoleucel, immunomodulatory agent, Janssen Biotech, proteasome inhibitor
Ciltacabtagene autoleucel is approved for relapsed or refractory multiple myeloma
March 2022: After four or more prior lines of therapy, including a proteasome inhibitor (PI), an immunomodulatory agent (IMiD), and an anti-CD38 monoclonal antibody, the Food and Drug Administration has approved ciltacabtagene au..
Amgen, carfilzomib, Darzalex Faspro, dexamethasone, Janssen Biotech, Kyprolis, multiple myeloma
Darzalex faspro, kyprolis, and dexamethasone is approved by FDA for multiple myeloma
March 2022: Daratumumab + hyaluronidase-fihj (Darzalex Faspro, Janssen Biotech, Inc.) and carfilzomib (Kyprolis, Amgen, Inc.) plus dexamethasone have been approved by the Food and Drug Administration for adult patients with relap..
Daratumumab, Darzalex Faspro, dexamethasone, Hyaluronidase-fihj, Janssen Biotech, pomalidomide
Daratumumab and hyaluronidase-fihj plus pomalidomide and dexamethasone have been approved by the FDA for the treatment of multiple myeloma
August 2021: Daratumumab and hyaluronidase-fihj (Darzalex Faspro, Janssen Biotech, Inc.) have been approved by the Food and Drug Administration in combination with pomalidomide and dexamethasone for adult patients with multiple my..