Braftovi with Erbitux finally achieved positive results for colorectal cancer
Targeted treatment of colorectal cancer, BRAF V600E gene mutation targeted therapy of colorectal cancer Braftovi + Erbitux finally achieved positive results
Colorectal cancer treatment status
Colorectal cancer is one of the most common malignant tumors in the digestive system. In recent years, its morbidity ranks third in the world in terms of malignant tumors, and its mortality rate ranks second, which seriously threatens people’s lives and health. With the changes in the living habits and dietary structure of our nationals, the incidence of colorectal cancer has generally shown an upward trend, and has become the second highest incidence of digestive system, and the highest incidence of malignant tumors. According to relevant research statistics, the number of new cases of colorectal cancer in China is expected to exceed 521,000 in 2018, and the number of deaths is as high as 248,000.
Studies have shown that about 15% of patients with metastatic colorectal cancer will have BRAF gene mutations and poor prognosis. V600E mutation is the most common BRAF gene mutation. The risk of death of patients with BRAF V600E mutation is to carry wild type BRAF gene Patients twice.
Faced with such a dangerous BRAF V600E mutation metastatic colorectal cancer, the editor shares a piece of exciting good news learned recently! On April 8, 2020, Pfizer announced that the US FDA has approved Braftovi® (encorafenib, connefenib) and Erbitux® (cetuximab, cetuximab) combination therapy (Braftovi two-drug protocol) is used to treat patients with metastatic colorectal cancer (mCRC) who carry the BRAF V600E mutation. These patients have already received one or two pre-treatments. This approval also makes the Braftovi second drug regimen the first targeted therapy approved by the FDA for patients with mCRC carrying BRAF mutations.
Braftovi double and triple therapy significantly prolongs survival
As early as December 2019, the FDA accepted the Pfizer Braftovi Second Drug Program’s supplementary new drug application and granted priority review qualification. This approval is based on the results of the BEACON CRC Phase 3 clinical trial.
The study was carried out in patients with advanced BRAF V600E mutant mCRC who had previously progressed after receiving one or two therapies. The efficacy and safety of ritica’s treatment plan combined with medication (control).
Table 1: The medication plan of each group
| Second medicine | Braftovi (encorafenib, Connefini) |
| Second medicine | Erbitux (cetuximab, cetuximab) |
| Three-drug program | Braftovi (encorafenib, Connefini) |
| Three-drug program | Erbitux (cetuximab, cetuximab) |
| Three-drug program | Mektovi (binimetinib, bemetinib) |
| Control group | Erbitux (cetuximab, cetuximab) |
| Control group | Irinotecan or FOLFIRI (folinic acid, fluorouracil and irinotecan) |
The main research results
1. Median survival (OS): 9.0 months in the triple therapy group
8.4 months in the dual therapy group
Control group is 5.4 months
2. Progression-free survival: 4.3 months in the triple therapy group
4.2 months for the dual therapy group
Control group is 1.5 months
3. 6-month survival rate: 71% in triple therapy group
65% in the dual therapy group
Control group is 47%
4. Objective Remission Rate (ORR): 26% in triple therapy group
20% in the dual therapy group
Control group is 2%
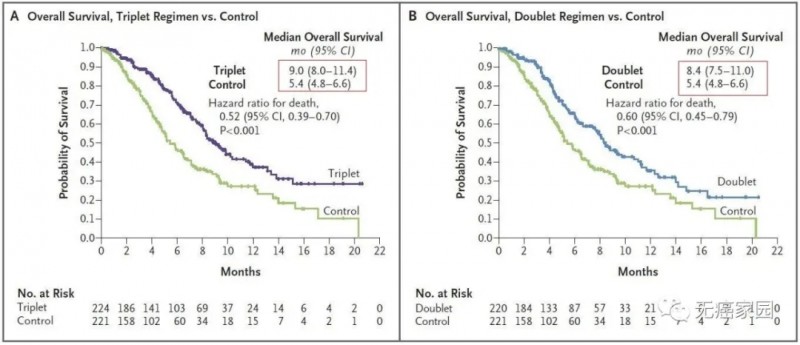
The picture on the left compares the three-drug regimen of raftovi with the OS of the control group, and the picture on the right compares the two-drug regimen of raftovi with the OS of the control group
In general, compared with Erbitux and irinotecan-containing treatment regimens, the efficacy of the two-drug regimen and the three-drug regimen is not much different, and there are fewer clinical side effects.
Principal Investigator Dr. Scott Kopetz said: “As a patient with previously treated BRAF V600E mutant metastatic colorectal cancer, Braftovi + Erbitux (conefinil + cetuximab) is the first and only targeted therapy This is the new treatment option that such patients badly need. “
Braftovi combination therapy principles and indications
Braftovi’s active pharmaceutical ingredient binimetinib is an oral small molecule BRAF inhibitor, and Mektovi’s active pharmaceutical ingredient encorafenib is an oral small molecule MEK inhibitor. MEK and BRAF are two key protein kinases in the MAPK signaling pathway (RAS-RAF-MEK-ERK).
Studies have shown that this pathway regulates many key cell activities including cell proliferation, differentiation, survival, and angiogenesis. In many cancers, such as melanoma, colorectal cancer, and thyroid cancer, proteins in this signaling pathway have been shown to be abnormally activated.
In the United States, the Braftovi + Mektovi combination has been approved for unresectable or metastatic melanoma with BRAF V600E or BRAF V600K mutations. Braftovi is not suitable for the treatment of wild-type BRAF melanoma. In Europe, the combination is approved for adults with unresectable or metastatic melanoma with BRAF V600 mutation. In Japan, the combination is approved for BRAF-mutated unresectable melanoma.
| English name | Chinese name | Target | manufacturer | Indications | Medicare |
| Trametinib (Mekinist) | Trametinib | MEK | Novartis (outside) | Same as above | no |
| Vemurafenib (Zelboraf) | Verofinil (Verofinil, Zuobofu) | BRAF | Roche Gold and Silver Tektronix (outside) | Melanoma | Yes, included in health insurance |
| Cobimetinib (Cotellic) | Cobitinib | MEK | Roche Gold and Silver Tektronix (outside) | Same as above | no |
| Encorafenib (Braftovi) | Connefini | BRAF | Array BioPharma | Melanoma | no |
| Binimetinib (Mektovi) | Bemetinib | MEK | Array BioPharma | Same as above | no |
The 2019 NCCN Guidelines for Colorectal Cancer adds two new EGFR / BRAF / MEK triple inhibitor combination therapies for patients with advanced BRAF V600E mutation-positive advanced disease, namely:
[1] Dabrafenib + Trametinib + Cetuximab / Panitumumab (Cetuximab / Panitumumab)
[2] Encorafenib (conefinil) + Binimetinib (bimetinib) + Cetuximab / Panitumumab (cetuximab / panitumumab)
Xiaobian has something to say
In the era of targeted therapy, every patient with colorectal cancer should pass MSI detection, mutation analysis of RAS and BRAF, and perform HER2 amplification, NTRK and other gene detection as far as possible. Genetic testing (NGS) will be included in the large The initial examination standard for most patients. Cancer friends who have undergone genetic testing can send the report to the medical department for interpretation to see if there is a relevant treatment option.
The editor believes that in the future there will be more recent research progress and the best medications for colorectal cancer. Only the top cancer experts at home and abroad have rich clinical experience. Colorectal cancer patients can apply for authority through the Global Oncologist Network Expert consultation, get the best diagnosis and treatment plan.
Susan Hau is a distinguished researcher in the field of cancer cell therapy, with a particular focus on T cell-based approaches and cancer vaccines. Her work spans several innovative treatment modalities, including CAR T-cell therapy, TIL (Tumor-Infiltrating Lymphocyte) therapy, and NK (Natural Killer) cell therapy.
Hau's expertise lies in cancer cell biology, where she has made significant contributions to understanding the complex interactions between immune cells and tumors.
Her research aims to enhance the efficacy of immunotherapies by manipulating the tumor microenvironment and exploring novel ways to activate and direct immune responses against cancer cells.
Throughout her career, Hau has collaborated with leading professors and researchers in the field of cancer treatment, both in the United States and China.
These international experiences have broadened her perspective and contributed to her innovative approach to cancer therapy development.
Hau's work is particularly focused on addressing the challenges of treating advanced and metastatic cancers. She has been involved in clinical trials evaluating the safety and efficacy of various immunotherapy approaches, including the promising Gamma Delta T cell therapy.
- Comments Closed
- May 18th, 2020



CancerFax is the most trusted online platform dedicated to connecting individuals facing advanced-stage cancer with groundbreaking cell therapies.
Send your medical reports and get a free analysis.
🌟 Join us in the fight against cancer! 🌟
Привет,
CancerFax — это самая надежная онлайн-платформа, призванная предоставить людям, столкнувшимся с раком на поздних стадиях, доступ к революционным клеточным методам лечения.
Отправьте свои медицинские заключения и получите бесплатный анализ.
🌟 Присоединяйтесь к нам в борьбе с раком! 🌟