Latest news in cancer
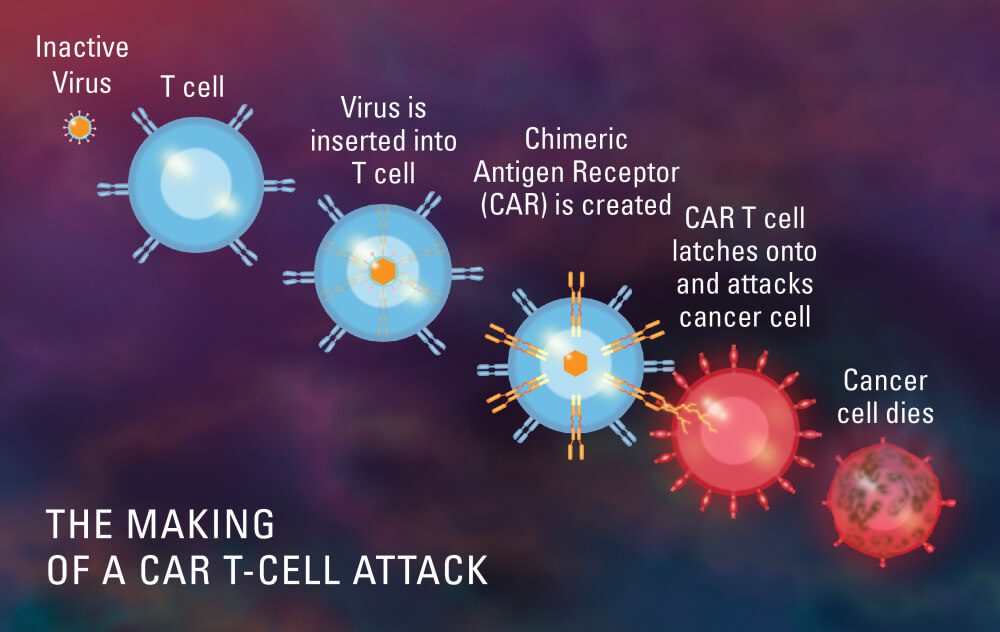
CT103A, CAR T-cell Therapy, has been designated as an orphan drug by the FDA
Feb 2023: The FDA has granted orphan drug status to CT103A, an experimental CAR T-cell therapy being developed by IASO Biotherapeutics and Innovent Biologics...
CAR T-cell treatment from IASO Biotherapeutics receives new FDA approval
Feb 2023: IASO Biotherapeutics’ investigational CAR T-cell therapy for relapsed or refractory multiple myeloma (RRMM), CT103A, has received fast track...
Dostarlimab-gxly is approved by FDA for dMMR endometrial cancer
Feb 2023: Dostarlimab-gxly (Jemperli, GlaxoSmithKline LLC) was given FDA approval to treat adult patients with mismatch repair deficient (dMMR) recurrent or...
Sacituzumab govitecan-hziy is approved by FDA for HR-positive breast cancer
Feb 2023: The Food and Drug Administration (FDA) has approved sacituzumab govitecan-hziy (Trodelvy, Gilead Sciences, Inc.) for people with hormone receptor...
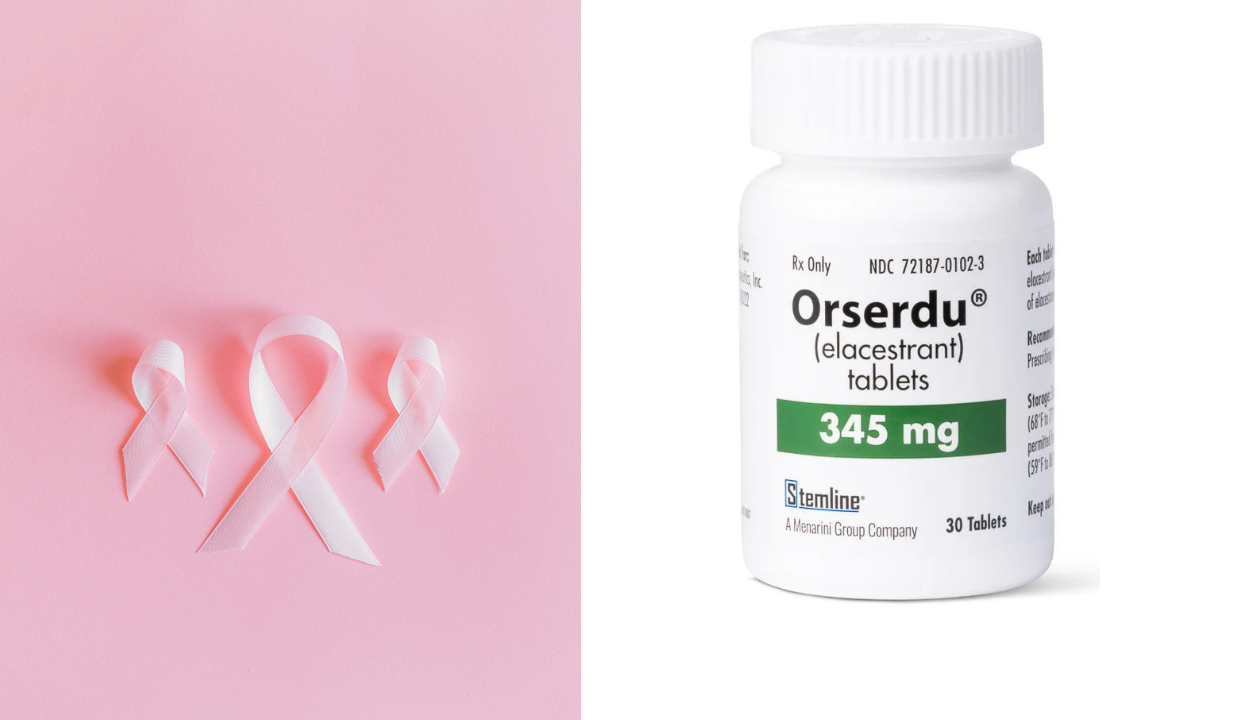
Elacestrant is approved by FDA for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer
In February 2023, the Food and Drug Administration (FDA) approved elacestrant (Orserdu, Stemline Therapeutics, Inc.) for women or men over 50 who have advanced...
Accelerated approval is granted by FDA to pirtobrutinib for relapsed or refractory mantle cell lymphoma
Feb 2023: Accelerated approval is granted by FDA to pirtobrutinib (Jaypirca, Eli Lilly and Company) for relapsed or refractory mantle cell lymphoma. In BRUIN...
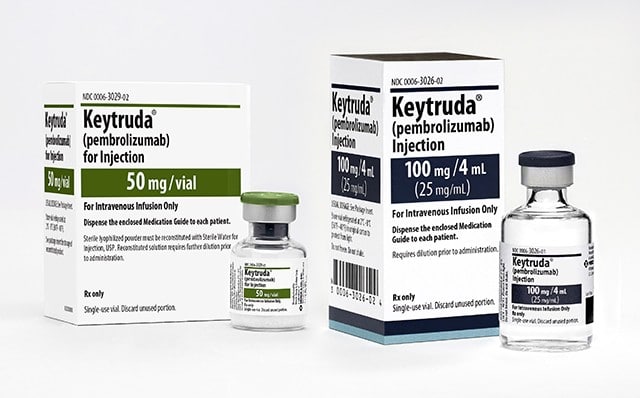
Pembrolizumab is approved by FDA as adjuvant treatment for non-small cell lung cancer
Feb 2023: For stage IB (T2a 4 cm), stage II, or stage IIIA non-small cell lung cancer, the Food and Drug Administration (FDA) approved pembrolizumab (Keytruda,...
Zanubrutinib is approved by FDA for chronic lymphocytic leukemia or small lymphocytic lymphoma
Feb 2023: Zanubrutinib (Brukinsa, BeiGene USA, Inc.) is approved by FDA for chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). SEQUOIA was...
Accelerated approval is granted by FDA to tucatinib with trastuzumab for colorectal cancer
In February 2023, the Food and Drug Administration (FDA) sped up the approval of tucatinib (Tukysa, Seagen Inc.) and trastuzumab for the treatment of RAS...
Bone marrow transplant in Iran
Bone marrow transplant is one of the services of Cancerfax, which is provided by the best surgeons, along with accommodation, a translator, a companion nurse,...
Sheba medical center became the first Israeli facility to receive permission from Medtronic International to train medical professionals on MICRA pacemaker implants
RAMAT GAN, Israel – June, 2022 – Sheba Medical Center, Israel’s largest medical center and a Newsweek top-10 ranked world’s best hospital for the last...
Utilization of wearable telehealth technology for remote patient monitoring is validated by new Sheba medical center study
July 2022: Peer-reviewed study analyzes usability of a wearable RPM device, which detected early warning of risk for ABCNO deterioration in 75% of...
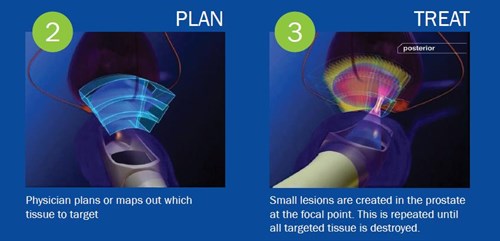
Focal HIFU therapy in prostate cancer
What does local prostate cancer mean? In Liv Hospital Urology Clinic, HIFU is applied as the primary treatment approach in localized prostate cancer, that...
AMC opens up CAR T-Cell therapy center in Seoul
Jan 2023: The Asan Medical Center (AMC) opened the first CAR-T cell treatment facility in the country after the government approved health insurance benefits...
Best cancer hospitals in India in 2023
Several notable cancer hospitals in India are known for their superior medical competence and cutting-edge technology. The Tata Memorial Hospital in Mumbai is...
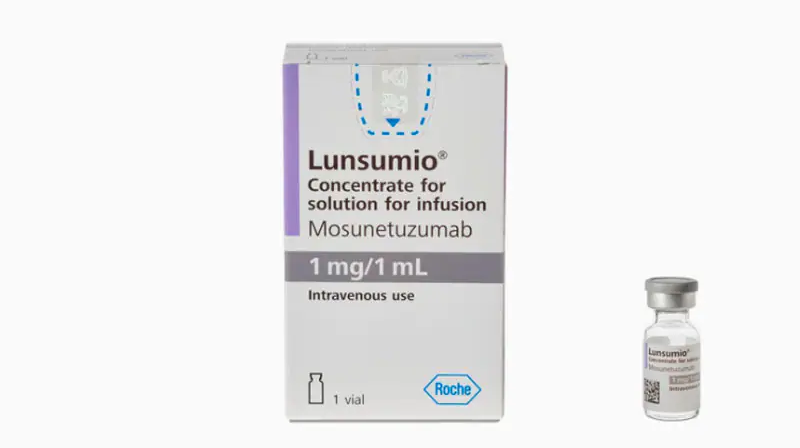
Mosunetuzumab-axgb is granted accelerated approval for relapsed or refractory follicular lymphoma
Jan 2023: Mosunetuzumab-axgb (Lunsumio, Genentech, Inc.), a bispecific CD20-directed CD3 T-cell engager for adult patients with relapsed or refractory...
First adenoviral vector-based gene therapy for high-risk Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer is approved by FDA
Jan 2023: The drug nadofaragene firadenovec-vncg (Adstiladrin, Ferring Pharmaceuticals) has been approved by the Food and Drug Administration for adult...
Adagrasib gets accelerated approval for KRAS G12C-mutated NSCLC
Jan 2023: Adagrasib (Krazati, Mirati Therapeutics, Inc.), a RAS GTPase family inhibitor, was given accelerated approval by the Food and Drug Administration...
Atezolizumab is approved by FDA for alveolar soft tissue sarcoma
Dec 2022: Atezolizumab (Tecentriq, Genentech, Inc.) has been approved by the Food and Drug Administration (FDA) for adult and paediatric patients with...
Olutasidenib is approved by FDA for relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation
Dec 2022: Olutasidenib (Rezlidhia) capsules were approved by the Food and Drug Administration (FDA) for adult patients with relapsed or resistant acute...
New dosing regimen for asparaginase erwinia chrysanthemi (recombinant) is approved by FDA
Dec 2022: A new Monday-Wednesday-Friday dosing schedule for asparaginase erwinia chrysanthemi (recombinant)-rywn has been approved by the Food and Drug...
Mirvetuximab soravtansine-gynx is granted accelerated approval for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer
November 2022: For adult patients who have had one to three prior systemic treatment regimens and have folate receptor alpha (FR) positive, platinum-resistant...
Tremelimumab is approved by FDA in combination with durvalumab and platinum-based chemotherapy for metastatic non-small cell lung cancer
November 2022: The combination of tremelimumab (Imjudo, AstraZeneca Pharmaceuticals), durvalumab (Imfinzi, AstraZeneca Pharmaceuticals), and platinum-based...
Brentuximab vedotin is approved by FDA in combination with chemotherapy for pediatric patients with classical Hodgkin lymphoma
November 2022: The combination of doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide with brentuximab vedotin (Adcetris, Seagen, Inc.) has...
Cemiplimab-rwlc is approved by FDA in combination with platinum-based chemotherapy for non-small cell lung cancer
November 2022: The combination of cemiplimab-rwlc (Libtayo, Regeneron Pharmaceuticals, Inc.) and platinum-based chemotherapy for adult patients with advanced...
Teclistamab-cqyv is approved by FDA for relapsed or refractory multiple myeloma
November 2022: The first bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager, teclistamab-cqyv (Tecvayli, Janssen Biotech, Inc.), was given...
Tremelimumab is approved by FDA in combination with durvalumab for unresectable hepatocellular carcinoma
November 2022: The Food and Drug Administration approved tremelimumab (Imjudo, AstraZeneca Pharmaceuticals) in combination with durvalumab for adult patients...
Cccelerated approval is granted by FDA to futibatinib for cholangiocarcinoma
November 2022: The Food and Drug Administration granted accelerated approval to futibatinib (Lytgobi, Taiho Oncology, Inc.) for adult patients with previously...
Selpercatinib is approved by FDA for locally advanced or metastatic RET fusion-positive solid tumors
November 2022: Selpercatinib (Retevmo, Eli Lilly and Company) received accelerated approval from the Food and Drug Administration to treat adult patients with...
Sodium thiosulfate is approved by FDA to reduce the risk of ototoxicity associated with cisplatin in pediatric patients with localized, non-metastatic solid tumors
November 2022: In children aged one month and older with localised, non-metastatic solid tumours, the Food and Drug Administration has approved sodium...
Durvalumab is approved for locally advanced or metastatic biliary tract cancer
November 2022: For adult patients with locally advanced or metastatic biliary tract cancer, the Food and Drug Administration has approved durvalumab (Imfinzi,...
Pemigatinib is approved for relapsed or refractory myeloid/lymphoid neoplasms with FGFR1 rearrangement
November 2022: Pemigatinib (Pemazyre, Incyte Corporation) has been licenced by the Food and Drug Administration for use in people with relapsed or refractory...
Ibrutinib is approved for pediatric patients with chronic graft versus host disease, including a new oral suspension
Sept 2022: Ibrutinib (Imbruvica, Pharmacyclics LLC) was approved by the Food and Drug Administration for use in paediatric patients with chronic graft versus...
Tecartus granted European marketing authorization for treatment of relapsed or refractory acute lymphoblastic leukemia
Sept 2022: The European Commission (EC) has authorized Kite’s CAR T-cell therapy Tecartus® (brexucabtagene autoleucel) for the treatment of adult...
CAR T-Cell therapy complications can be predicted by a simple blood test
Sept 2022: The treatment of various tumours has been transformed by cell-based immunotherapy, often known as CAR-T cell therapy. In order to target and combat...
New test to determine whether CAR T-Cell therapy will work for lymphoma patients
Sept 2022: According to a study published in the Journal of Clinical Investigation, an engineer at the University of Houston (UH) may have discovered a...
Need help? Our team is ready to assist you.
We wish a speedy recovery of your dear and near one.