What does local prostate cancer mean?
In Liv Hospital Urology Clinic, HIFU is applied as the primary treatment approach in localized prostate cancer, that is, at the stage where the entire cancer is in the prostate tissue and the surrounding tissues are intact.
Prostate cancer can be encountered by chance during surgery
Incidentally, prostate cancer can be seen in 12% of patients who undergo open or endoscopic surgery with the diagnosis of benign prostatic hyperplasia, that is, BPH-benign prostatic enlargement. These patients need additional treatment for cancer, but conventional treatments may cause them to undergo local prostate cancer treatment with more severe complications. Tumor-focused Focal HIFU treatment in primary prostate cancer can provide patients with an uncomplicated process.
What is Focal HIFU?
HIFU is a current treatment method applied as local treatment in primary prostate cancer, as a salvage treatment after radiotherapy and surgical failure, and as a supportive treatment in locally advanced prostate cancer. “Radical HIFU” when integrated with TUR is applied as focal HIFU when non-invasive apart from TUR. Considering the entire treatment process of prostate cancer, which is a variable and long-term disease, HIFU is a versatile treatment technique. HIFU cannot be compared with any classical treatment method, but its indications may overlap with all other treatments throughout the course of the disease and may create alternatives. In addition, it can be applied to patients of all ages and all health conditions due to the fact that the procedure can be performed in a single session and the side effects are low during and after the procedure, as well as because of its non-invasiveness.
For which patients is Focal HIFU treatment suitable?
Overtreatment is seen in prostate cancer. The need for less invasive and adequate treatments is very high. For this reason, it is preferable to apply this type of treatment strategy to patients with a single focal low-risk tumor in the prostate.
The aim is to plan a partial and tumor-limited treatment strategy without TUR in unifocal, localized prostate cancer cases. In the event of failure or relapse of this type of treatment, there is a chance of total/radical transformation. On the one hand, it is aimed at protect sphincter function and sexual performance. On the other hand, in the wait-see situation, the psychological stress that the patient will experience is relieved. Against the question of “overtreatment,” focal treatment of prostate cancer is a non-invasive method.
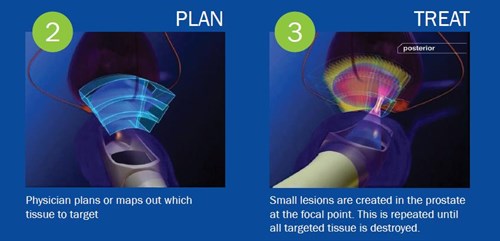
How is Focal HIFU treatment applied?
HIFU is a non-invasive treatment performed under general anesthesia and completed in a single session. In the procedure, an ultrasonic scanner is used, which focuses the ultrasonographic waves emitted by a spoon-shaped applicator, which is placed in the rectum and contains an angled piezoelectric crystal. The HIFU firing sequence, intensity, and duration of the applicators are specific to each case. The intrarectal position of the applicators during the procedure is determined in 3D with a computerized algorithm, the measurements are checked with a 3D image, corrected, and automatic and instant real-time ultrasonic imaging is performed for each lesion according to the treatment plan. Thus, the highest intraoperative acuity is provided in the HIFU application. This is the feature that makes the technologies that apply the HIFU process “intelligent surgical robot”.