Gene Therapy for Thalassemia
Gene Therapy Applied to Thalassemia: An Overview
Thalassemia is one such blood disorder characterized by the body being unable to produce enough hemoglobin, making life unusually challenging for both patients and healthcare providers. Traditional treatment options have involved regular blood transfusions and iron chelation therapy; however, these treatment regimens are fraught with complications and are often lifelong commitments. Recent developments in gene therapy open new avenues toward a causal and promising treatment for this disorder. Gene therapy for thalassemia is the most promising development in the treatment of this genetic disorder.
What is thalassemia?
Thalassemia segregates into two major types: alpha and beta thalassemia. Accordingly, it is due either to mutations in the HBA1 and HBA2 genes, called alpha thalassemia, or to mutations in the HBB gene, called beta thalassemia. Beta thalassemia is divided into beta-thalassemia minor, intermedia, and major, the last of which is the most severe form, also called Cooley’s anemia.
Gene Therapy for Thalassemia: Mechanism and Action
Gene therapy for thalassemia is the process of introducing copies of the affected gene into the function of hematopoietic stem cells of the patients. Several gene therapy approaches are already in place for thalassemia:
- Gene Addition Therapy: This is a process by which a normal copy of the abnormal gene is put into the stem cells of the patient using viral vectors. The edited stem cells are taken back to the bone marrow of the patient, where from they will start to produce healthy red blood cells.
- Gene Editing: These techniques involve making changes directly to the genomic level that would be expected to repair or rectify the mutation. One such recent technique is CRISPR-Cas9. It has already evoked much interest because of its high degree of specificity and efficiency.
- Genome Regulation: It counterbalances defective adult hemoglobin by inducing modulation in the genes of hemoglobin production, including that of fetal hemoglobin.
Why choose our gene therapy?
Best in class
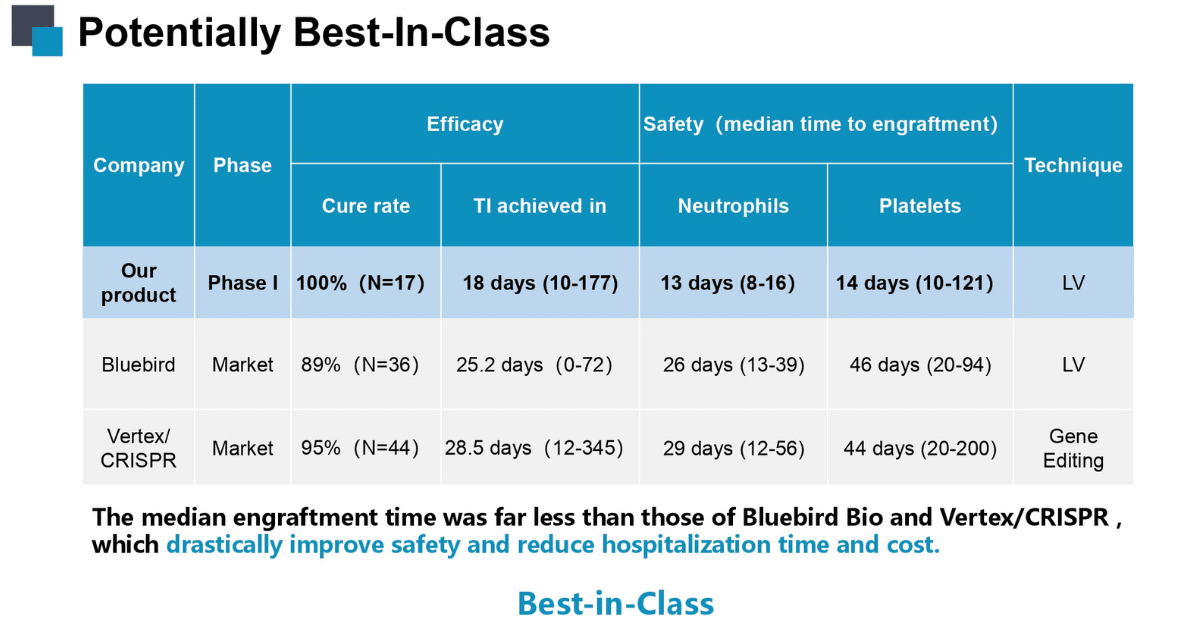
Safety and Efficacy
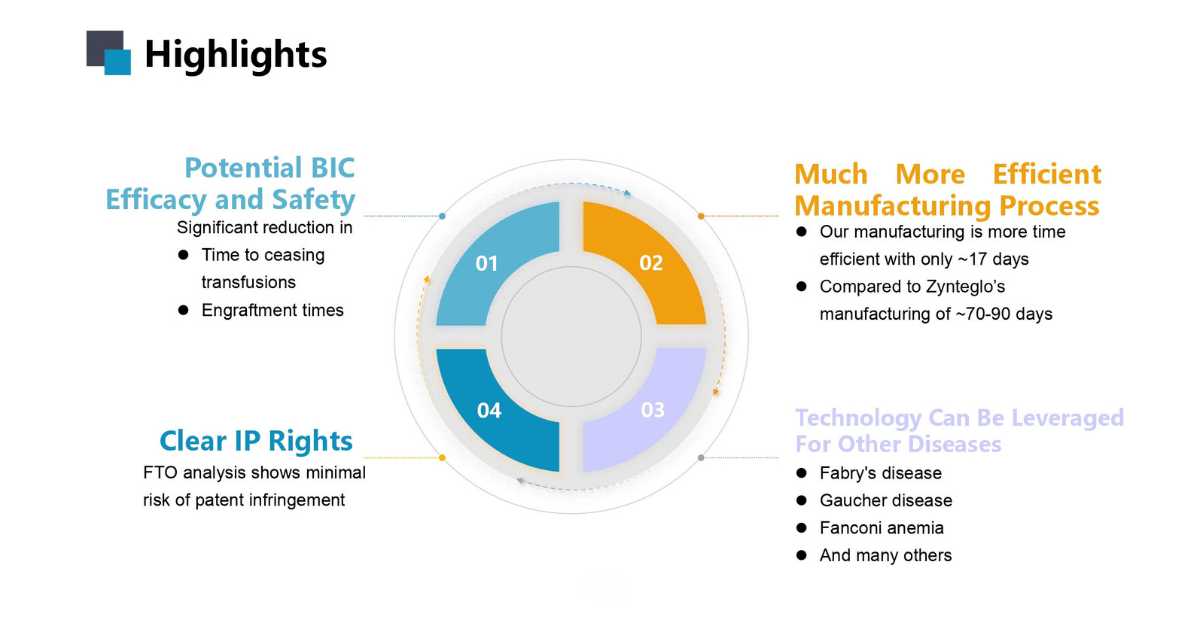
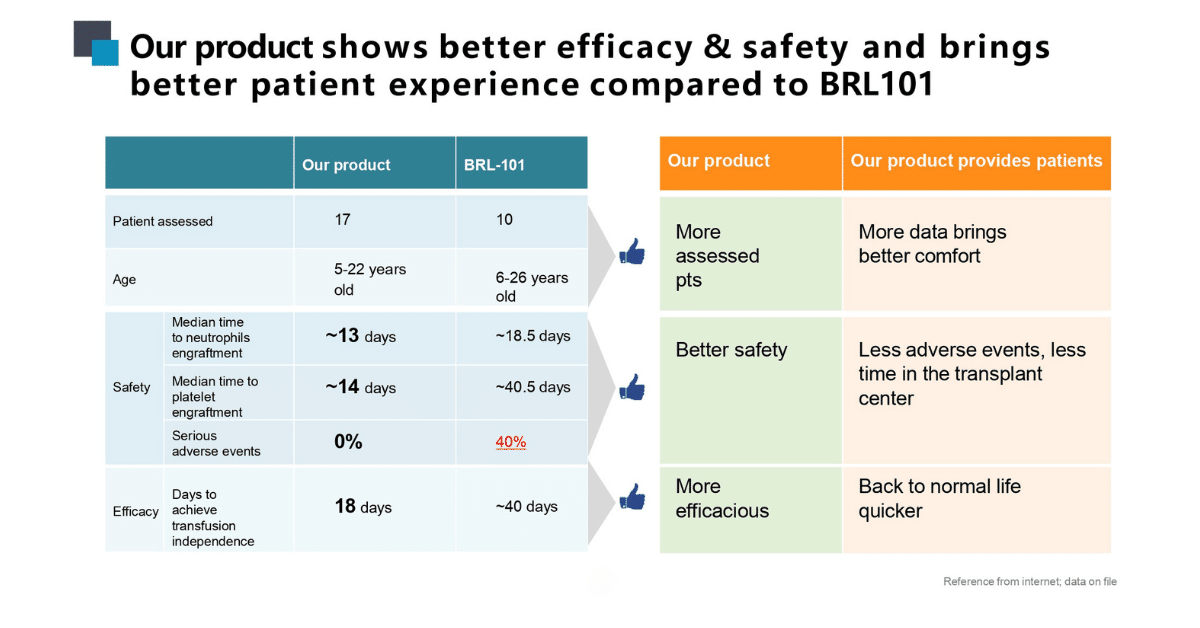
Along with better efficacy and safety, our product brings better quality of life to the patient
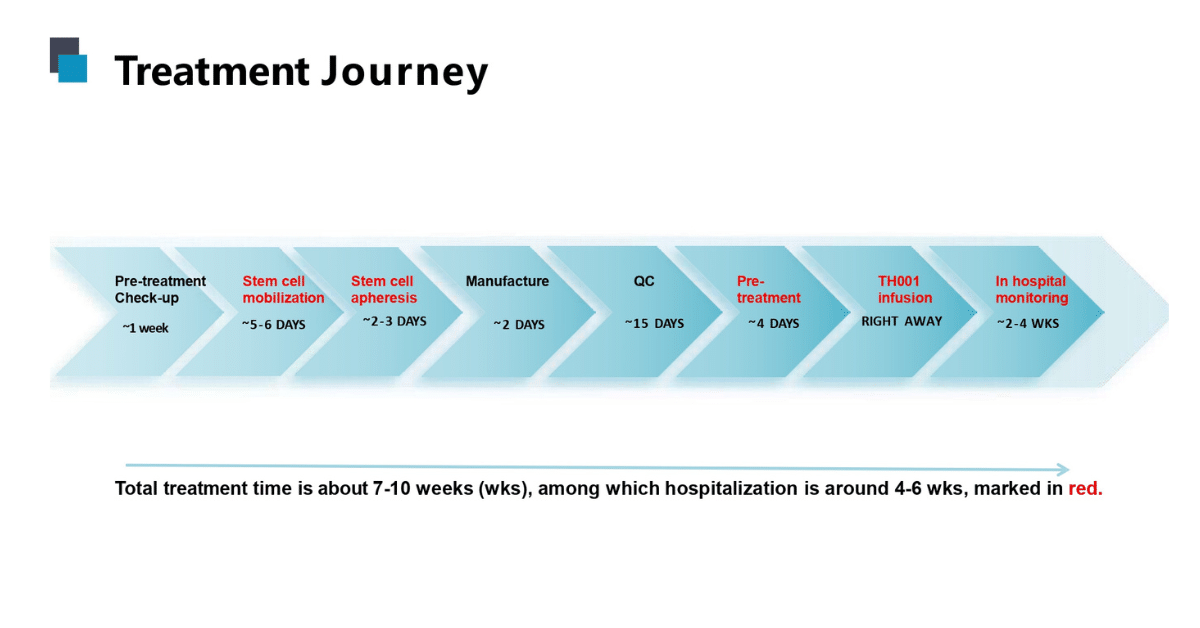
Gene therapy treatment process and time lines
FDA-approved gene therapy for beta-thalassemia
The first gene therapy for beta-thalassemia, Zynteglo, has received FDA approval in a land-marking development. This gene therapy aims at introducing the patient’s stem cells to a functional copy of the HBB gene through a lentiviral vector. Clinical trials, however, have been quite successful, with approximately 90% of patients achieving transfusion independence.
Gene Therapy for Thalassemia in China
The country with the highest prevalence of thalassemia is China, where gene therapy would really make a difference. There are also efforts to make gene therapy treatments available to patients from China. So, the cost of gene therapy in China becomes one important aspect in this situation. It will bring down the cost, making the therapies more easily accessible. Various clinical trials and research projects in this context are going on in different hospitals and institutions, such as Beijing Gobroad Hospital, Beijing Cancer Hospital.
Cost of Gene Therapy for Thalassemia
Gene therapy, although advanced, is also one of the most expensive treatments currently available. Gene therapy for thalassemia in developed countries would cost approximately from $ 1 million to $ 2 million. In China, this could be lower but still be high enough to make a hole in anyone’s pocket. Amongst the plans available to bring the cost down and closer to the ground are the optimization of production procedures, an increase in competition and regulatory support. Gene therapy in China will cost around $ 175,000 USD at present. Hope this will come down considerably in the coming months.
Updated Perspectives of Gene Therapy on Beta-Thalassemia
Recent studies and trials update the perspectives on efficacy and safety of this approach in the treatment of beta-thalassemia. Long-term follow-ups support continued benefits with gene therapy, such as transfusion independence, significant improvement of overall quality of life. However, issues, include potential insertional mutations and the need for conditioning regimens.
Gene Therapy for Alpha Thalassemia
Whereas the majority of studies have been devoted to beta thalassemia, it is also demonstrated for gene treatment of alpha thalassemia; the method is pretty much the same—either addition or correction of the HBA1 and HBA2 genes. Decent implementation of gene therapy in alpha thalassemia, therefore, will significantly improve the treatment options for this subtype of patients.
Gene Therapy and Gene Editing for β-Thalassemia
Gene editing, represented by CRISPR-Cas9, has changed the face of gene therapy in thalassemia. The new gene editing techniques offer Substantial accuracy in correcting specific mutations in the HBB gene in β-thalassemia. Recent improvements in gene editing have achieved high efficiency with reduced off-target effects, substantially increasing the potential of this technology for clinical applications.
Gene Therapy for Thalassemia Major
Thalassemia major exhibits the most severe symptoms and complications, so it stands out as the most appropriate candidate for gene therapy. Trials with gene therapy revealed that transfusion independence is possible for patients with thalassemia major, subsequently improving quality of life and reducing the risks associated with regular blood transfusions.
Future Directions and Challenges
Several issues remain to be addressed before gene therapy becomes a more mainstream treatment for thalassemia:
1. Safety and Efficacy: Long-term safety and efficacy are very important for gene therapy. Monitoring will have to be done on a continuous basis through follow-up studies in order to recognize risks and minimize them.
2. Accessibility and Cost: Gene therapy has to reach more people, particularly in low- and middle-income countries. There are endeavors in progress to bring the cost down and raise the capacity of production.
3. Regulatory Approvals: Approaching regulatory authorities for approval of gene therapy requires several clinical data points and close coordination with the regulatory dissension.
4. Public Awareness: Increased public awareness about gene therapy is required for such advanced therapy to be supported and accepted.
Conclusion
Gene therapy for thalassemia is the most promising development in the treatment of this genetic disorder. With therapies such as Zynteglo already approved and ongoing efforts in research, the horizon holds out some promise for those afflicted with beta and alpha thalassemias. Gene therapy has the potential to cure thalassemia; hence, millions of patients around the world can get hope. Still, it is clear that there are big challenges to follow. In the second place, and more importantly, when ongoing research remains dynamic, it becomes very imperative that these therapies are made safe and effective and that they are accessible to all in need.
Dr. Nishant Mittal is a highly accomplished researcher with over 13 years of experience in the fields of cardiovascular biology and cancer research. His career is marked by significant contributions to stem cell biology, developmental biology, and innovative research techniques.
Research Highlights
Dr. Mittal's research has focused on several key areas:
1) Cardiovascular Development and Regeneration: He studied coronary vessel development and regeneration using zebrafish models1.
2) Cancer Biology: At Dartmouth College, he developed zebrafish models for studying tumor heterogeneity and clonal evolution in pancreatic cancer.
3) Developmental Biology: His doctoral work at Keio University involved identifying and characterizing medaka fish mutants with cardiovascular defects.
4) Stem Cell Research: He investigated the effects of folic acid on mouse embryonic stem cells and worked on cryopreservation techniques for hematopoietic stem cells.
Publications and Presentations
Dr. Mittal has authored several peer-reviewed publications in reputable journals such as Scientific Reports, Cardiovascular Research, and Disease Models & Mechanisms1. He has also presented his research at numerous international conferences, including the Stanford-Weill Cornell Cardiovascular Research Symposium and the Weinstein Cardiovascular Development Conference.
In summary, Dr. Nishant Mittal is a dedicated and accomplished researcher with a strong track record in cardiovascular and cancer biology, demonstrating expertise in various model systems and a commitment to advancing scientific knowledge through innovative research approaches.
- Comments Closed
- July 12th, 2024





Beta thalassemia cure, CRISPR for thalassemia, Curative options for thalassemia, Gene editing for anemia, Genetic treatment for blood disorders, Hemoglobin disorder treatment, Thalassemia gene therapy, Zynteglo therapy
🌟 Welcome to Beijing Biotech! 🌟
Thank you for reaching out! We are dedicated to transforming lives through advanced gene therapy solutions for sickle cell disease and thalassemia. Our team is committed to delivering cutting-edge treatments, personalized care, and support on every step of your journey to better health.
Please feel free to ask any questions or let us know how we can assist you. Together, we’re here to make a difference.
Warm regards,
The Beijing Biotech Team