First gene therapy approved by the USFDA to treat sickle cell disease
USFDA approved gene therapy for sickle cell disease
The U.S. Food and Drug Administration has granted approval for two significant medications, Casgevy and Lyfgenia, which are the first gene therapies using cell-based techniques to treat sickle cell disease (SCD) in patients aged 12 and above. Moreover, Casgevy, one of these medicines, is a pioneering FDA-approved medication that employs a unique genome editing technology, representing a groundbreaking progress in the domain of gene therapy.
Sickle cell disease
Sickle cell disease is a collection of hereditary blood abnormalities that impact around 100,000 individuals in the United States. It is predominantly observed in African Americans and, although less widespread, also impacts Hispanic Americans. The main issue in sickle cell disease is a genetic mutation in hemoglobin, a protein present in red blood cells responsible for transporting oxygen to the body’s tissues. This genetic mutation results in the formation of red blood cells that have a crescent or “sickle” shape. Sickled red blood cells cause vaso-occlusive episodes (VOEs) or vaso-occlusive crises (VOCs) by constricting blood vessels and reducing oxygen delivery to the body’s tissues. This leads to extreme pain and organ damage. The repetition of these occurrences or emergencies can result in life-endangering impairments and/or premature mortality.
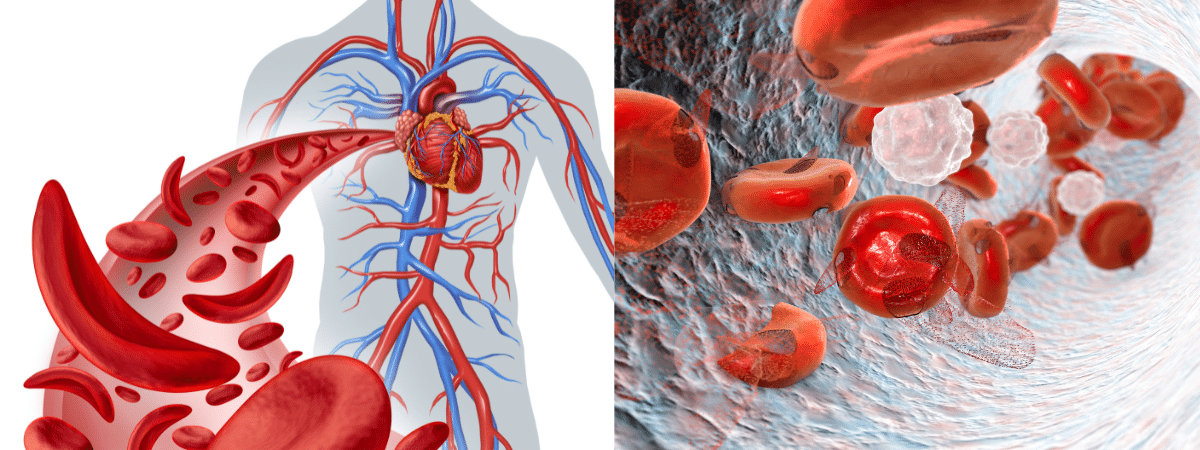
Pic: Sickle cell disease
“Sickle cell disease is a rare and serious blood disorder that poses a significant challenge. For those whose lives the disease has severely impacted, we are enthusiastic about making progress in this area. Today, we have approved two gene therapies that use cells to treat the condition,” stated Nicole Verdun, M.D., who serves as the director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research. Gene therapy offers the potential to provide more precise and efficient treatments, particularly for those with uncommon diseases that have few available therapeutic choices.
CRISPR/Cas9 technology
The cell-based gene therapy known as Casgevy has received approval for treating sickle cell disease in people aged 12 and above who have recurring vaso-occlusive crises. The first drug using CRISPR/Cas9 genome editing technology is Casgevy, according to the FDA. Hematopoietic stem cells of patients are genetically edited utilizing CRISPR/Cas9 technology.
The CRISPR/Cas9 system may be specifically guided to cleave DNA at desired locations, allowing for precise manipulation of DNA by removing, adding, or replacing genetic material at the site of cleavage. The altered blood stem cells are reintroduced into the patient’s body, where they attach and reproduce in the bone marrow.
This leads to an augmentation in the synthesis of fetal hemoglobin (HbF), a specific form of hemoglobin that aids in the transportation of oxygen. Elevated levels of HbF in individuals with sickle cell disease inhibit the process of red blood cell sickling.
Casgevy

A gene-editing treatment called Casgevy, which used to be called CTX001 or exa-cel, is approved for people with sickle cell disease (SCD) who have repeated vaso-occlusive crises (VOCs). Its purpose is to decrease the frequency and intensity of these agonizing episodes.
The treatment, delivered by a single intravenous infusion, was developed by CRISPR Therapeutics and Vertex Pharmaceuticals. Casgevy is approved to treat transfusion-dependent beta thalassemia (TDT) in addition to sickle cell disease (SCD).
Lyfgenia
Lyfgenia is a form of gene therapy that utilizes cells as its primary method of treatment. Lyfgenia employs a lentiviral vector, which is a vehicle for delivering genes, to carry out genetic modification. It has been authorized for the treatment of individuals aged 12 and above who have sickle cell disease and a past record of vaso-occlusive episodes. Lyfgenia is a treatment that involves modifying the patient’s blood stem cells to create HbAT87Q, a kind of hemoglobin derived from gene therapy.
This modified hemoglobin behaves in a similar way to hemoglobin A, which is the typical adult hemoglobin seen in individuals without sickle cell disease. Red blood cells that have HbAT87Q exhibit a reduced propensity for sickling and obstructing blood flow. Subsequently, the altered stem cells are administered to the individual.
Both products are derived from the patients’ autologous hematopoietic stem cells, which undergo modification and are then administered as a singular infusion during a hematopoietic stem cell transplant. Before treatment, the patient’s own stem cells are gathered, and then the patient must undergo myeloablative conditioning, which involves administering high-dose chemotherapy.
This process eliminates cells from the bone marrow to make way for the modified cells in Casgevy and Lyfgenia. Long-term research will be conducted to assess the safety and efficacy of Casgevy and Lyfgenia in patients who have received these medications.
“These approvals signify a significant medical breakthrough in utilizing cutting-edge cell-based gene therapies to specifically target potentially catastrophic diseases and enhance public health,” stated Peter Marks, M.D., Ph.D., the director of the FDA’s Center for Biologics Evaluation and Research. “The actions taken today are based on thorough evaluations of scientific and clinical data required for approval, demonstrating the FDA’s dedication to promoting the development of safe and effective treatments for conditions that have significant impacts on human health.”
Evidence in Favor of Casgevy
Casgevy’s safety and effectiveness were assessed in an ongoing trial including multiple centers, where adult and adolescent patients with SCD were included. Patients had a documented record of experiencing at least two protocol-defined severe vaso-occlusive crises (VOCs) in each of the two years before the screening. The main measure of effectiveness was the absence of severe VOC episodes for a minimum of 12 consecutive months within the 24-month follow-up period. There were a total of 44 individuals that received treatment with Casgevy. Out of the 31 patients that were able to be evaluated for a sufficient amount of time, 29 of them (93.5%) obtained the desired outcome. All patients who received treatment had effective engraftment, with no instances of graft failure or graft rejection seen.
The predominant adverse effects seen included thrombocytopenia and leukopenia, oral ulcers, emesis, musculoskeletal discomfort, stomach discomfort, febrile neutropenia, cephalalgia, and pruritus.
Evidence in Favor of Lyfgenia
The safety and efficacy of Lyfgenia is established by the examination of data from a 24-month multicenter research involving patients with sickle cell disease with a history of vaso-occlusive events (VOEs) who are between the ages of 12 and 50 years old. The effectiveness of Lyfgenia was assessed by determining the complete resolution of VOEs (VOE-CR) during a timeframe of 6 to 18 months following the infusion. During this time period, 88% (28 out of 32) of the patients obtained complete resolution of VOE.
Stomatitis (inflammation of the lips, mouth, and throat), thrombocytopenia (low platelet count), leukopenia (low white blood cell count), and anemia (low red blood cell count) were the main side effects that were seen. These are common side effects of chemotherapy and the underlying medical condition.
Patients who have been treated with Lyfgenia have developed hematologic malignancy, which is a type of blood cancer. The label for Lyfgenia contains a black box warning that provides information about this risk. Patients who are prescribed this medicine should undergo continuous monitoring throughout their lives for the development of various types of cancer.
Susan Hau is a distinguished researcher in the field of cancer cell therapy, with a particular focus on T cell-based approaches and cancer vaccines. Her work spans several innovative treatment modalities, including CAR T-cell therapy, TIL (Tumor-Infiltrating Lymphocyte) therapy, and NK (Natural Killer) cell therapy.
Hau's expertise lies in cancer cell biology, where she has made significant contributions to understanding the complex interactions between immune cells and tumors.
Her research aims to enhance the efficacy of immunotherapies by manipulating the tumor microenvironment and exploring novel ways to activate and direct immune responses against cancer cells.
Throughout her career, Hau has collaborated with leading professors and researchers in the field of cancer treatment, both in the United States and China.
These international experiences have broadened her perspective and contributed to her innovative approach to cancer therapy development.
Hau's work is particularly focused on addressing the challenges of treating advanced and metastatic cancers. She has been involved in clinical trials evaluating the safety and efficacy of various immunotherapy approaches, including the promising Gamma Delta T cell therapy.
- Comments Closed
- July 13th, 2024






Casgevy approval, CRISPR treatment for sickle cell, Exa-cel therapy, FDA-approved cure for SCD, First CRISPR-approved drug, Genetic cure for anemia, Sickle cell breakthrough treatment, Sickle cell gene therapy
🌟 Welcome to Beijing Biotech! 🌟
Thank you for reaching out! We are dedicated to transforming lives through advanced gene therapy solutions for sickle cell disease and thalassemia. Our team is committed to delivering cutting-edge treatments, personalized care, and support on every step of your journey to better health.
Please feel free to ask any questions or let us know how we can assist you. Together, we’re here to make a difference.
Warm regards,
The Beijing Biotech Team