
CAR T-Cell Therapy in Korea
Utilizing cutting-edge biomedical research, South Korean medical facilities have spearheaded groundbreaking CAR T cell therapies, ushering in a new era of cancer treatment. These innovative interventions cater to patients grappling with hematologic malignancies and solid tumors, showcasing South Korea’s prowess in personalized medicine.
By tailoring CAR T cell treatments to the unique characteristics of each patient, medical centers in South Korea not only boost therapeutic outcomes but also mitigate potential side effects. This progressive methodology highlights South Korea’s dedication to propelling oncological healthcare forward.
South Korea is paying a lot of attention to CAR T-cell therapy, which is a new kind of immunotherapy that has made a lot of progress. It involves changing the genes of a patient’s own T cells so they can make chimeric antigen receptors (CARs), which can find cancer cells and kill them. South Korea has done a lot of research and development on CAR T cell therapy, with a number of clinical trials and treatment centres devoted to this new method. CAR T cell therapy has been successful in South Korea because of the country’s healthcare system and technological knowledge. This has given patients with blood cancers hope and paved the way for more personalized cancer treatments in the region.
CAR T-Cell therapy in Korea – Recent advances
June 2023 : CAR T-cell therapy has become a new way to treat cancer. It has changed the way we fight different types of cancer. In recent years, South Korea has made a lot of progress in this area, which shows how dedicated it is to study and new ideas. This piece will talk about some of the new developments in CAR T-cell therapy in Korea. It will focus on the ground-breaking changes that have put South Korea at the top of this field.
Clinical Trials and Regulatory Approval: South Korea has actively pursued clinical trials for CAR T cell therapy, creating an atmosphere that encourages innovation and care that focuses on the patient. The country has been at the forefront of testing treatments for haematological diseases and solid tumours, among other types of cancer. Notably, clinical trials have shown positive results, which led to CAR T cell therapies being approved by the government for some uses.
Development of New CAR Constructs: Researchers and scientists in South Korea have been working hard to make new CAR constructs that make CAR T cell treatment more effective and safer. This includes making improvements to the way chimeric antigen receptors are made so that CAR T cells can better find, activate, and stay in tumours. These changes have led to better treatment results and fewer side effects.
Combination Therapies: South Korean researchers have also looked into the possibility of mixing CAR T cell therapy with other types of treatment, such as immune checkpoint inhibitors and targeted therapies. Researchers want to improve response rates and get around problems caused by the different kinds of tumours and how they hide from the immune system.
Manufacturing and Commercialization: South Korea has made a lot of progress in creating processes for making CAR T cell treatments that are strong and can be scaled up. This has made it possible for these treatments to be put on the market and used by more people, making them easier for people who need them to get.
South Korea is now the world’s leader in CAR T cell therapy. It has done this by using its research skills, clinical knowledge, and regulatory framework to make amazing progress in this area. The country’s dedication to innovation and its collaborative research environment have led to the creation of new CAR constructs, successful clinical trials, and the commercialization of these therapies. These new developments have a lot of potential to change how cancer is treated and improve patient results, not just in South Korea but all over the world. South Korea is likely to have a big impact on the future of CAR T cell treatment as the field continues to change.
CAR T-cell therapy, developed by Novartis and marketed under the brand name Kymriah, is currently being provided by the CAR T-cell Therapy Center to patients under the age of 25 who suffer from B-cell acute lymphoblastic leukaemia or diffuse large B-cell lymphoma. In addition, patients who have refractory diffuse large B-cell lymphoma are the focus of clinical trials being conducted for CRCO1, which was developed by a company based in Korea called Curocell. In addition, because Korea is widely recognised as the CAR T-cell Therapy hub in the world, clinical trials for the CAR T-cell therapy developed by Janssen are also currently being conducted for patients who have relapsed or refractory multiple myeloma.
Before beginning treatment, patients undergoing CAR T-cell therapy are required to coordinate their efforts with a number of different hospital departments. To be more specific, a large number of specialists in fields that are closely related to infectious disease, neurology, cardiology, and intensive care are involved in the comprehensive management that takes place after treatment.
The CAR T-cell therapy centre is currently participating in the “Research-oriented Hospital R&D Project” by the Ministry of Health and Welfare, and it is expected to become a cell therapy centre that leads high-level cell therapy and patient-specific treatment for haematological oncology patients. The project was initiated by the Ministry of Health and Welfare.
Which companies are involved in manufacturing CAR T-Cells in South-Korea?
On November 16, 2022, ViroMed, a Korean firm, announced a plan to develop a CAR-T drug. CAR gene optimization technology, vector production technology, and ex vivo technology for gene transfer and cell proliferation are all available to the business for the development of CAR-T drugs. After selling “VM801,” ViroMed’s own CAR-T technology, to US Bluebird Bio for 56.8 billion won in December 2015, the company plans to produce three CAR-T products.
In addition, AbClon is developing a blood cancer treatment based on technology developed by a research team led by professor Jung Jun-ho of Seoul National University in February, which is safer than current CAR-T drugs. The team’s technology is known to distinguish itself from current drugs by greatly reducing cytokine release syndrome (CRS), a detrimental side effect of CAR-T drugs that can cause hypotension, fever, and even death in patients.
To top it off, Green Cross Cell, a subsidiary of Green Cross, is screening and validating CAR-T drug candidates for solid cancer treatment. The company intends to conduct research in order to begin a pre-clinical trial next year. Since naming Son Ji-woong as head of the Life Science Business Division in February, LG Chem is said to have been contemplating the implementation of technology related to CAR-T drugs, with the production of diabetes drugs and immunological anti-cancer drugs being the company’s key R&D mission.
Curocell, a South Korean biotech startup that pioneered CAR-T cell therapy, is planning to launch a clinical trial of a novel CAR-T cell therapy that targets both blood cancer and solid tumours.
Curocell recently submitted an IND application for a clinical trial of its CAR-T cell therapy in Korea, and the company’s chief executive Kim Gun-soo told Maeil Business Newspaper that the Ministry of Food and Drug Protection is expected to approve it this month.
Curocell has already formed a strategic alliance with Samsung Medical Center to kick-start the trial as soon as it is approved, according to Kim, who hopes to get the groundbreaking cancer therapy to Korean patients as soon as possible.
CAR-T is an immunotherapy that is programmed to identify and destroy cancer cells. T cells from a single patient are genetically engineered in a lab to produce cancer-specific chimeric antigen receptors in order to create this treatment. After that, the cancer-fighting cells are re-infused into the patient’s body.
CAR-T cell therapy has been shown to be more successful than any other cancer treatment currently available. According to Kim, despite failing other therapies, 82 percent of acute leukaemia patients and 32-36 percent of lymphoma patients became cancer-free with a single CAR-T cell infusion.
However, CART’s high efficiency results are limited to the treatment of blood cancer cells and cannot be replicated for solid tumours due to the immune checkpoint receptor (PD-1) found in solid cancer patients’ immune cells, which inhibits immune cell activity.
The immune checkpoint receptor serves as a brake on immune cells’ ability to function. When immune checkpoint receptors like PD-1 are overexpressed, immune cells’ ability to remove cancer cells decreases, making tumour removal more difficult.
Curocell’s OVIS (Overcome Immune Suppression) technology overcomes this restriction, allowing CAR-T cell therapy to be used to treat solid tumours. OVIS is a genetic engineering technology that uses genetic modification of immune cells to suppress immune checkpoint receptor RNA (ribonucleic acid). A new CAR-T cell therapy implemented with this technology was more successful than a current CAR-T treatment in an animal model, according to Kim.
Except for Curocell’s therapy, none of the CAR-T cell therapies that are currently available or in clinical trials will target both blood cancer and solid tumours, according to Curocell.
How to apply for CAR T-Cell therapy in Korea?
Send your medical reports to info@cancerfax.com or WhatsApp them to +1-213 789-56-55. Send following reports for opinion and estimate:
1) Medical summary
2) Latest blood reports
3) Biopsy
4) Latest PET Scan
5) Bone marrow biopsy (If available)
6) Any other relevant reports and scans
Once our team receives your medical reports, we analyze them and send it to hospitals that are performing CAR T-Cell therapy with that type of cancer and marker. We send reports to the concerned specialist and get his opinion. Report analysis is followed by an online video consultation with the specialist. We also get estimate from the hospital on complete treatment. This helps you in planning for the entire treatment duration.
Once you decide to visit for the treatment, we arrange for medical visa letter and other necessary documents from the hospital. We also help and guide you in applying for medical visa to the Korean embassy. Once visa is ready we help and guide you in preparing for travel and flight tickets. We also arrange for your hotel and guest house, if required in South-Korea. Upon arrival in the city of treatment our representative will welcome you at the airport.
Our representative will arrange for doctor appointment and complete the necessary registration formalities for you. He will also help you with your hospital admission and other local help and support that are required. After the treatment is over we will arrange for your follow up consultation with the treating doctor.
Top hospitals for CAR T-Cell therapy in Korea

Asan medical center
South Korea’s Asan Medical Centre has been at the forefront of using new technologies to treat blood cancer, with the goal of improving patient results and quality of life. The centre has invested in cutting-edge technologies like next-generation sequencing (NGS) for accurate genetic mapping of tumours, which makes it possible to treat each patient individually. Asan Medical Centre has also added new treatments, such as CAR T cell therapy, which uses the patient’s immune system to attack cancer cells. These tools have changed how blood cancer is found, tracked, and treated, giving patients new hope and making Asan Medical Centre a leader in the fight against blood cancer.
What is the cost of CAR T-Cell therapy in Korea?
Cost of CAR T-Cell therapy in South-Korea starts from $ 450,000 USD and can reach up to $ 500,000 USD depending upon the type and extent of disease and its burden on the body. It is crucial to keep in mind that this estimate is only a general approximation and that specific circumstances may change. The precise type of CAR-T therapy, the intricacy of the procedure, hospital costs, supportive care, and additional diagnostic testing are all variables that can affect price. To control the costs related to CAR-T cell therapy, it is advised for patients to speak with healthcare professionals and look into insurance coverage or financial help programmes.
What is CAR T-Cell therapy?
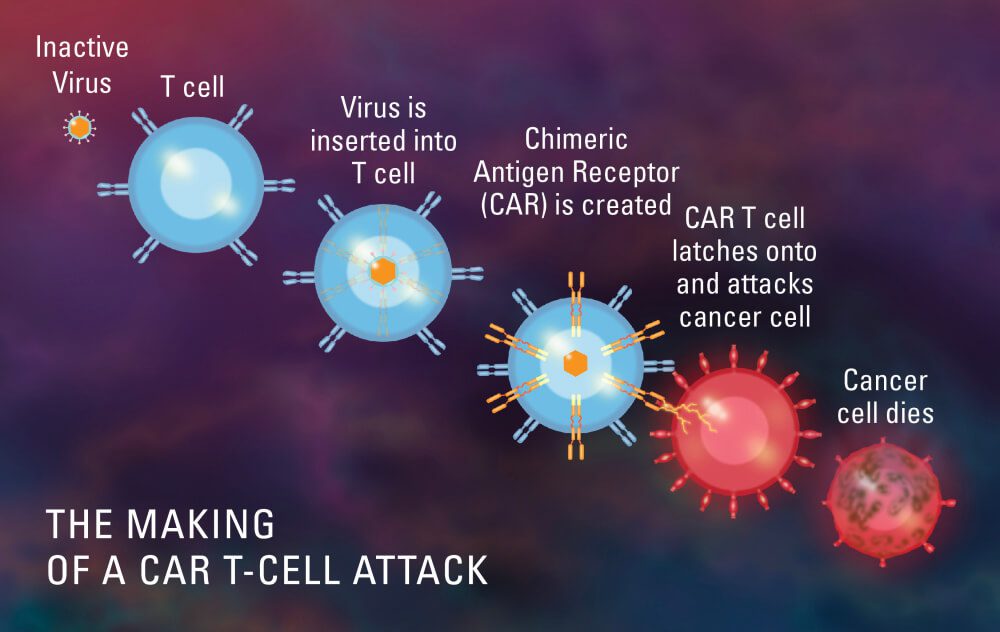
Chimeric antigen receptor T-cell therapy, often known as CAR T-cell therapy, is a ground-breaking immunotherapy that has completely changed the way that cancer is treated. It gives patients with certain cancers hope that were previously seen as incurable or with few therapeutic alternatives.
The treatment entails using a patient’s own immune cells—more specifically, T cells—and lab-modifying them to improve their capacity to detect and destroy cancer cells. To do this, the T cells are given a chimeric antigen receptor (CAR), which gives them the ability to target particular proteins, or antigens, on the surface of cancer cells.
T cells from the patient are first removed, and they are then genetically modified to express the CAR. In the laboratory, these altered cells are multiplied to produce a sizable population of CAR T cells, which are then put back into the patient’s bloodstream.
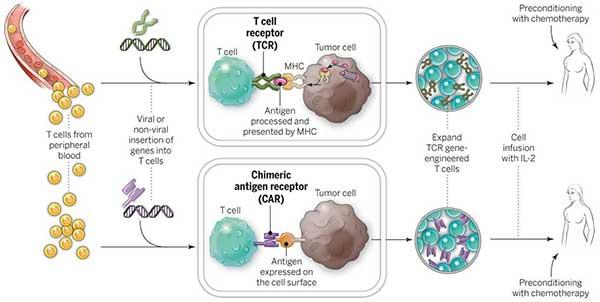
As soon as they are inside the body, CAR T cells find cancer cells that express the desired antigen, attach to them, and trigger a potent immune response. The CAR T cells that have been activated proliferate and conduct a focused attack on the cancer cells, killing them.
How does CAR T-Cell therapy work?
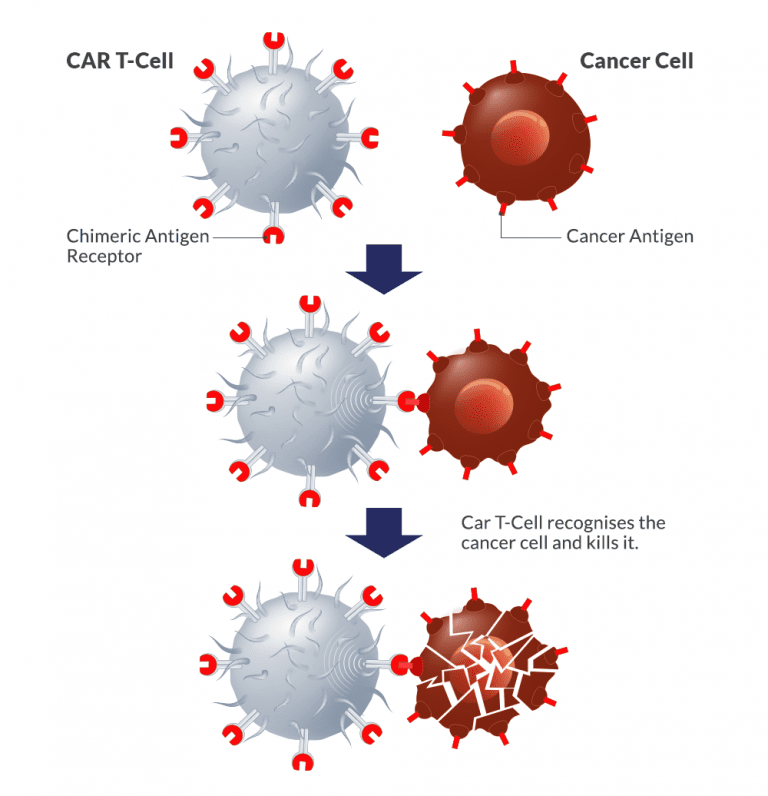
When used to treat some blood malignancies like acute lymphoblastic leukaemia (ALL) and specific forms of lymphoma, CAR T-cell therapy has shown exceptional results. It has produced notable response rates and in some patients, even long-lasting remissions.
CAR T-cell therapy, however, is a sophisticated and unique therapeutic method that might have risks and adverse effects. Cytokine release syndrome (CRS), a widespread immunological reaction that can result in flu-like symptoms and, in extreme situations, organ failure, may be experienced by certain people. There have also been reports of neurological adverse effects, however they are frequently curable.
Despite these difficulties, CAR T-cell therapy is a significant advancement in the fight against cancer and shows great potential for the future. Current studies are focused on enhancing its efficacy and safety profile as well as extending its uses to different cancer types. CAR T-cell therapy has the ability to change the face of cancer treatment and give patients everywhere new hope with further advancements.
Procedure
The CAR-T therapy procedure, which takes a few weeks, involves multiple steps:
T cells are extracted from your blood using a tube that is placed into an arm vein. This takes a couple of hours.
T cells are transported to a facility where they undergo genetic modification to become CAR-T cells. Two to three weeks pass throughout this.
CAR-T cells are reintroduced into your bloodstream through a drip. This requires several hours.
CAR-T cells target and eliminate cancer cells throughout the body. After receiving CAR-T therapy, you will be closely watched.
What type of cancer cells can be treated with CAR-T Cell Therapy?
Only patients with adult B-cell non-lymphoma Hodgkin’s or pediatric acute lymphoblastic leukemia who have already tried two unsuccessful conventional therapies can currently use CAR T-cell therapy products that have received FDA approval. However, CAR T-cell therapy is now being tested in clinical studies as a first or second-line treatment for adult lymphoma and pediatric acute lymphoblastic leukemia. Recently, some of the studies have shown remarkable successes in cases of solid tumors too like glioblastoma, gliomas, liver cancer, lung cancer, GI cancer, pancreatic cancer and oral cancers.
To conclude
This represents a significant advancement in the management of leukemia and B-cell lymphoma. Additionally, it gives hope to those whose lives had previously been predicted to last only six months. Now that we have identified mechanisms of resistance and created more techniques to combat them, the future appears to be much more promising.
Get in touch with our highly experienced healthcare providers here at CancerFax for a free consultation to work out a suitable care plan for your healthcare needs. Please send your medical reports to info@cancerfax.com or WhatsApp to +1 213 789 56 55.
What are the advantages of CAR-T Cell Therapy?
The main benefit is that CAR T-cell therapy only requires a single infusion and often only requires two weeks of inpatient care. Patients with non-Hodgkin lymphoma and pediatric leukemia who have just been diagnosed, on the other hand, typically need chemotherapy for at least six months or more.
The advantages of CAR T-cell therapy, which is actually a living medication, can persist for many years. If and when a relapse occurs, the cells will still be able to identify and target cancer cells because they can survive in the body for an extended period of time.
Although the information is still developing, 42% of adult lymphoma patients who underwent CD19 CAR T-cell treatment were still in remission after 15 months. And after six months, two-thirds of patients with pediatric acute lymphoblastic leukemia were still in remission. Unfortunately, these patients had exceedingly aggressive tumors that weren’t successfully treated using traditional standards of care.
What type of patients would be good recipients of the CAR-T Cell Therapy?
Patients between the age of 3 Years to 70 Years have been tried with CAR T-Cell therapy for different type of blood cancers and has been found to be very effective. Many centers have claimed success rates of more than 80%. The optimum candidate for CAR T-cell therapy at this time is a juvenile with acute lymphoblastic leukemia or an adult with severe B-cell lymphoma who has already had two lines of ineffective therapy.
Before the end of 2017, there was no accepted standard of care for patients who had already gone through two lines of therapy without experiencing remission. The only FDA-approved treatment that has so far proven to be significantly beneficial for these patients is CAR T-cell therapy.
How effective is CAR-T Cell therapy?
CAR T-cell therapy has been very effective in treating some types of blood cancer, like acute lymphoblastic leukaemia (ALL) and non-Hodgkin lymphoma. In clinical trials, the response rates have been very good, and a lot of patients have gone into full remission. In some cases, people who had tried every other medicine had long-lasting remissions or even possible cures.
One of the best things about CAR T-cell treatment is that it targets the right cells. The CAR receptors that have been added to the T cells can find specific marks on cancer cells. This makes it possible to give targeted treatment. This targeted method hurts healthy cells as little as possible and lowers the risk of side effects that come with traditional treatments like chemotherapy.
But it’s important to keep in mind that CAR T-cell therapy is still a new area that is still changing. Researchers and doctors are working hard to solve problems like the high cost, the possibility of serious side effects, and the fact that it only works for some types of cancer.
In the end, CAR T-cell therapy has shown to be a very successful way to treat some types of blood cancer. Even though it is a promising and powerful method, more study and clinical trials are needed to improve it and find new ways to use it. CAR T-cell therapy could change how cancer is treated and make things better for people all over the world if it keeps getting better.
Inclusion & exclusion criteria
Inclusion criteria for CAR T-cell therapy:
1. Patients with CD19+ B-cell Lymphoma(At least 2 prior combination chemotherapy regimens)
2. To be aged 3 to 75 years
3. ECOG score ≤2
4. Women of childbearing potential must have a urine pregnancy test taken and proven negative prior to the treatment. All patients agree to use reliable methods of contraception during the trial period and until follow-up for the last time.
Exclusion criteria for CAR T-cell therapy:
1. Intracranial hypertension or unconsciousness
2. Respiratory failure
3. Disseminated intravascular coagulation
4. Hematosepsis or Uncontrolled active infection
5. Uncontrolled diabetes.
CAR T-Cell therapies approved by USFDA
B-cell precursor acute lymphoblastic leukemia, relapsed or refractory diffuse large B-cell lymphoma
Complete response rate (CR): >90%
Target: CD19
Price: $475,000
Approval time: August 30, 2017
Relapsed or refractory diffuse large B-cell lymphoma, relapsed or refractory follicular cell lymphoma
Non-Hodgkin’s lymphoma Complete response rate (CR): 51%
Target: CD19
Price: $373,000
Approval time: 2017 October 18
Relapsed or refractory diffuse large B-cell lymphoma
Mantle cell lymphoma Complete response rate (CR): 67%
Target: CD19
Price: $373,000
Approved time: October 18, 2017
Relapsed or refractory diffuse large B-cell lymphoma
Complete response rate (CR): 54%
Target: CD19
Price: $410,300
Approved time: October 18, 2017
Relapsed or Refractory Multiple Myeloma
Complete response rate: 28%
Target: CD19
Price: $419,500
Approved: October 18, 2017
What are the side effects of CAR-T Cell therapy?
Below mentioned are some of the side-effects of CAR T-Cell therapy.
- Cytokine release syndrome (CRS): The most prevalent and possibly significant side effect of CAR T-cell treatment is cytokine release syndrome (CRS). The flu-like symptoms, including fever, exhaustion, headaches, and muscle pain, are brought on by the modified T cells’ production of cytokines. In extreme circumstances, CRS may result in a high temperature, hypotension, organ failure, and even potentially fatal consequences.
- Neurological Toxicity: Some patients may develop neurological side effects, which can range in severity from less serious signs like mild confusion and disorientation to more serious ones like seizures, delirium, and encephalopathy. After CAR T-cell infusion, neurological toxicity frequently happens during the first week.
- Cytopenias: CAR T-cell treatment can result in low blood cell counts, such as anaemia (low red blood cell count), neutropenia (low white blood cell count), and thrombocytopenia (low platelet count). Infections, bleeding, and exhaustion are among risks that can be exacerbated by these cytopenias.
- Infections: The CAR T-cell therapy’s suppression of healthy immune cells increases the risk of bacterial, viral, and fungal infections. In order to prevent infections, patients may need to be closely watched and given preventive medication.
- Tumour Lysis Syndrome (TLS): After CAR T-cell therapy, it’s possible in some circumstances for substantial amounts of cell contents to be released into the bloodstream due to the rapid killing of tumour cells. This may result in metabolic abnormalities, such as excessive potassium, uric acid, and phosphate levels, which may damage the kidneys and cause other problems.
- Hypogammaglobulinemia: CAR T-cell treatment has the potential to decrease antibody synthesis, which could result in hypogammaglobulinemia. This might make recurrent infections more likely and call for continuing antibody replacement medication.
- Organ Toxicity: CAR T-cell therapy has the potential to harm a number of organs, including the heart, lungs, liver, and kidneys. This may lead to abnormal renal function tests, respiratory issues, heart issues, and abnormal liver function tests.
- Hemophagocytic lymphohistiocytosis (HLH): A rare but possibly fatal immunological disease called hemophagocytic lymphohistiocytosis (HLH) can develop as a result of CAR T-cell therapy. It involves the overactivation of immune cells, which causes serious organ damage and inflammation.
- Hypotension and Fluid Retention: As a result of the cytokines that CAR T cells release, some patients may develop low blood pressure (hypotension) and fluid retention. To address these symptoms, supportive measures including intravenous fluids and drugs could be required.
- Secondary Malignancies: Reports of secondary malignancies emerging following CAR T-cell therapy exist, notwithstanding their rarity. Research is currently being done on the potential for secondary malignancies and long-term hazards.
It’s important to remember that not every patient will have these side effects, and that each person’s level of sensitivity will differ. In order to minimize and minimize these potential adverse effects, the medical team closely examines patients before, during, and after CAR T-cell therapy.
Time frame
Check below the total time frame required for complete the CAR T-Cell therapy process. Although time frame depends a lot on the distance of lab from the hospital that prepared the CAR’s.
- Examination & test: one week
- Pre-treatment & T-Cell Collection: one week
- T-Cell preparation & return: two-three weeks
- 1st Effectiveness analysis: three weeks
- 2nd Effectiveness analysis: three weeks.
Total time frame: 10-12 Weeks