CAR T-Cell therapy for Chronic Lymphocytic Leukemia
Introduction
Chronic lymphocytic leukemia (CLL) is a long-lasting condition where mature B lymphocytes, which have encountered antigens before, become cancerous. This leads to the buildup of malignant B cells in the blood, bone marrow, lymph nodes, and spleen. Chronic lymphocytic leukemia (CLL) is the predominant form of leukemia in Western countries. The prevalence of chronic lymphocytic leukemia (CLL) in North America and Eastern Europe is 4.7 cases per 100,000 individuals annually. However, the incidence of CLL is significantly greater, reaching over 35 cases per 100,000 individuals in the population aged 85 years and older.
The clinical therapy of chronic lymphocytic leukemia (CLL) presents challenges and is contingent upon factors such as the patient’s age, presence of other medical conditions, and specific characteristics of CLL cells, including immunoglobulin heavy chain gene mutation, 17p deletion, TP53 mutation, and, as new studies have indicated, the quantity and specific kind of CLL tumor clones in the patient.
The treatment options for asymptomatic early-stage CLL range from a watch-and-wait approach to chemo-immunotherapy and novel targeted treatments, such as Bruton’s tyrosine kinase inhibitors and inhibitors of Bcl-2 family proteins, for symptomatic and advanced illness. Nevertheless, despite the proliferation of innovative therapeutic strategies, chronic lymphocytic leukemia (CLL) remains predominantly an untreatable condition.
Cell-based immunotherapy has recently become a new and innovative treatment for malignant disorders. One type of immunotherapy called cell-based immunotherapy uses immune cells taken from patients, grown in a lab, and genetically changed to make them better at finding and killing cancer cells (Figure 1). Cancer treatments based on T cells have been made, including lymphocytes that go into tumors, T cells that have had their TCRs changed, and cells that are able to recognize and attack cancer cells.
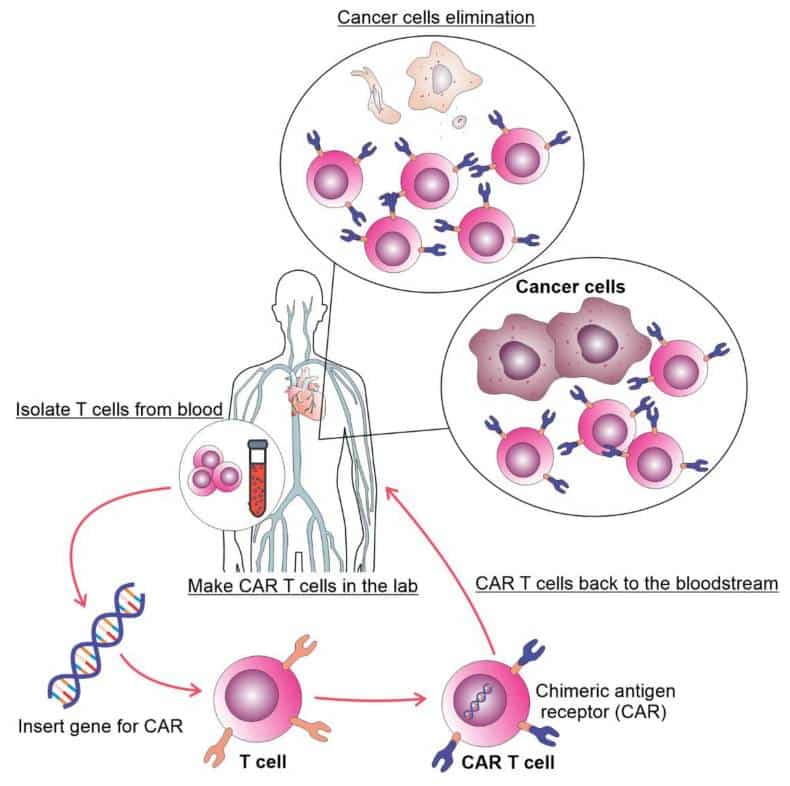
It has been shown that CAR T cells work better and last longer than tumor-infiltrating lymphocytes and TCR-modified T cells when used to treat cancer. To make CAR T cells, scientists changed the genes of T lymphocytes so that they have synthetic receptors that can find and kill cells that show a certain antigen. CARs are man-made proteins that contain the T-cell activation domain and a single-chain variable fragment of an antibody (ScFv) that specifically targets antigens in tumors.
Generations of CAR T-Cell therapy
Based on the T-cell activation domain found in the hybrid receptor, CAR T cells are categorized into four generations. The first version of CAR T cells only had the CD3 zeta domain attached to receptors that recognized a specific tumor antigen. The initial iteration of CAR T cells showed restricted therapeutic efficacy. In the second generation of CAR T cells, there are hybrid receptors that have CD3 and one of the other costimulatory molecules, like OX40, CD28, ICOS, CD-137/4-1BB, or CD28.
On the other hand, third-generation CAR T cells are created by combining two or more costimulatory molecules into the receptor sequence. The fourth generation of CAR T cells is designed with an inducible expression of cytokines that enhance the immune response against tumors. It also includes a self-withdrawal mechanism, which involves a suicide gene that may be activated once the desired anti-tumor effect is achieved. This gene prompts the quick withdrawal of CAR T cells.
Multiple challenges frequently accompany the favorable clinical outcomes of CAR T cell therapy in some cancers (Figure 2). Due to the high medication levels in most patients, there is a decrease in both the quantity and quality of T cells. As a result, it can be challenging to produce and expand autologous CAR T cells from these individuals. The production of allogeneic CAR T cells using healthy donors shows potential, but it can also lead to severe graft-versus-host disease (GvHD).
In spite of this, the discovery that allogenic NK cells are less likely to cause GvDH led to the creation of CAR immune cells. The presence of CAR constructs in CAR NK cells serves to activate the NK cells and enhance their natural capacity to eliminate cancerous cells.
First CAR T-Cell therapy to be approved by the USFDA
Bristol Myers Squibb (NYSE: BMY) has announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Breyanzi ® (lisocabtagene maraleucel; liso-cel), a type of therapy that uses modified immune cells called CAR T cells to target CD19, a protein found on cancer cells. Bruton tyrosine kinase (BTK) inhibitors and B-cell lymphoma 2 (BCL-2) inhibitors are two other treatments that adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) that have failed their first two can get this one.
This indication has been granted accelerated approval based on the response rate and duration of the response. Further approval for this specific use may depend on the confirmation and explanation of the clinical advantages in subsequent trials. In the context of R/R CLL or SLL, Breyanzi is administered to patients through a therapeutic procedure that concludes with a single infusion.
This infusion consists of a solitary dosage containing 90 to 110 x 106 CAR-positive viable T cells. Kindly refer to the section below titled “Important Safety Information” for detailed information on Breyanzi, including boxed warnings for Cytokine Release Syndrome (CRS), neurologic toxicities, and secondary hematological malignancies.
CAR T cell therapies offer a revolutionary treatment choice for individuals diagnosed with specific forms of blood cancer,” stated Bryan Campbell, the senior vice president and Head of Commercial for Cell Therapy at Bristol Myers Squibb. Efforts to introduce alternative CAR T cell therapies to individuals suffering from relapsed or refractory CLL or SLL have faced obstacles and achieved limited triumph.
By granting clearance to Breyanzi as the initial CAR T treatment for relapsed or refractory CLL or SLL, we can now provide these patients with a customized choice, while also increasing availability for a wide range of B-cell malignancies in order to address this urgent and unfulfilled requirement.
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are prevalent forms of B-cell lymphoma. Using targeted medicines, especially BTK- and BCL-2 inhibitors, is the main way that people with CLL or SLL are treated.
Nevertheless, patients frequently encounter relapse or develop resistance after initial therapy with these medicines, and there is currently no universally accepted treatment protocol for patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have been exposed to two different classes of drugs. Following a relapse or becoming resistant to these treatments, patients have few alternatives and experience unfavorable results, such as a lack of long-lasting full responses.
The TRANSCEND CLL 004 study was a Phase 1/2 clinical research that assessed the efficacy of CAR T cell treatment in patients with relapsed or refractory CLL or SLL. It was an open-label, single-arm study conducted at many centers and was the first of its kind. The complete response (CR) rate observed with Breyanzi therapy was 20%, with a 95% confidence interval (CI) ranging from 11.1% to 31.8%. The median length of response among patients who achieved a complete response (CR) was not determined at the time of data cutoff, with a 95% confidence interval ranging from 15 months to not reached (NR). Out of all the individuals that responded, the overall response rate (ORR) was 45%, with a 95% confidence interval (CI) ranging from 32.3% to 57.5%. The median length of response was 35.3 months, with a 95% CI ranging from 12.4 months to not reported (NR). The patients treated with Breyanzi who obtained a complete response (CR) showed high rates of minimum residual disease (MRD) negative status. The MRD-negativity rate was 100% in the blood (95% CI: 75.3-100) and 92.3% in the bone marrow (95% CI: 64-99.8).
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are currently regarded as diseases that cannot be cured and have limited treatment options when they come back after treatment. These treatment options can lead to complete responses, which have historically been linked to better long-term outcomes,” stated Tanya Siddiqi, M.D., the main researcher and Associate Professor in the Division of Lymphoma at City of Hope National Medical Center.
The FDA’s approval of liso-cel for people with relapsed or refractory CLL and SLL who have already been treated with BTKi and BCL2i is a big step forward. This approval marks a shift in the treatment approach, moving away from continuous therapy with sequential regimens to a personalized T-cell based approach. This new approach has the potential to provide patients with complete and long-lasting remission, overcoming drug resistance.
The study included 89 individuals who were treated with Breyanzi, and the incidence of cytokine release syndrome (CRS) and neurologic events (NEs) was predominantly of a low severity. CRS of any grade was observed in 83% of patients, while Grade 3 CRS was observed in 9% of patients. There were no documented events of Grade 4/5 CRS. NEs of any grade were reported in 46% of patients, with Grade 3 NEs observed in 20% of patients and one case of Grade 4 NE documented. There were no reported NEs for Grade 5.
According to Dr. Brian Koffman, a physician, CLL patient, co-founder, executive vice president, and chief medical officer of CLL Society, there are few therapeutic options available for individuals who are experiencing a relapse or have refractory CLL or SLL.
Patients with relapsed or refractory CLL or SLL now have new hope thanks to the approval of Breyanzi as the first CAR T cell therapy. After a single infusion, these patients may experience long-lasting responses. We express our gratitude to the patients and their families who participate in the studies, as well as to all the researchers who contributed to the development of this significant new therapy option for CLL and SLL.

Bristol Myers Squibb provides a range of services and resources to meet the needs of patients and caregivers. They offer help to ensure access to medicines, including Breyanzi. Bristol Myers Squibb additionally enhances the patient and physician treatment experience through the provision of Cell Therapy 360, a digital service platform that improves access to pertinent information, manufacturing updates, and assistance for patients and caregivers.
About TRANSCEND CLL 004
Transcend CLL 004 (NCT03331198) is a Phase 1/2 open-label, single-arm, multicenter study that looks at Breyanzi in people who have chronic lymphocytic leukemia or small lymphocytic lymphoma that has returned or stopped responding to treatment. The Phase 1 dose escalation portion of the study assessed the safety and recommended dose for the subsequent Phase 2 expansion cohort.
The Phase 2 portion of the study is evaluating Breyanzi at the recommended dose from the Phase 1 monotherapy arm. The main goal of Phase 2 of the study is to find the percentage of people who have a complete response, which includes those who have gone into complete remission but not yet fully recovered their bone marrow. This will be decided by an independent review committee following the guidelines set by the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) in 2018.
About Breyanzi
Breyanzi is a CD19-directed CAR T cell therapy that has a 4-1BB costimulatory domain that helps the CAR T cells grow and stay in the body longer. Breyanzi is made from a patient’s own T cells, which are collected and genetically reengineered to become CAR T cells that are then delivered via infusion as a one-time treatment.
Breyanzi is approved in the U.S., Japan and Europe for the second-line treatment of relapsed or refractory LBCL, and in Japan, Europe, Switzerland, and Canada for relapsed and refractory LBCL after two or more lines of systemic therapy. Bristol Myers Squibb’s clinical development program for Breyanzi includes clinical studies in other types of lymphoma. For more information, visit clinicaltrials.gov.
* Treatment process includes leukapheresis, manufacturing, administration, and adverse event monitoring.
Indication
BREYANZI is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
- adult patients with large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B, who have:
- refractory disease to first-line chemoimmunotherapy or relapse within 12 months of first-line chemoimmunotherapy; or
- first-line chemo-immunotherapy does not work or the disease comes back after first-line chemoimmunotherapy, and the person is too old or has other health problems to be a candidate for hematopoietic stem cell transplantation (HSCT); or
- relapsed or refractory disease after two or more lines of systemic therapy.
Limitations of Use: BREYANZI is not indicated for the treatment of patients with primary central nervous system lymphoma.
- Adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have had at least two previous lines of treatment, such as a Bruton tyrosine kinase (BTK) inhibitor and a B-cell lymphoma 2 (BCL-2) inhibitor, and whose cancer has returned or is not responding to treatment.
This indication is approved under accelerated approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
Susan Hau is a distinguished researcher in the field of cancer cell therapy, with a particular focus on T cell-based approaches and cancer vaccines. Her work spans several innovative treatment modalities, including CAR T-cell therapy, TIL (Tumor-Infiltrating Lymphocyte) therapy, and NK (Natural Killer) cell therapy.
Hau's expertise lies in cancer cell biology, where she has made significant contributions to understanding the complex interactions between immune cells and tumors.
Her research aims to enhance the efficacy of immunotherapies by manipulating the tumor microenvironment and exploring novel ways to activate and direct immune responses against cancer cells.
Throughout her career, Hau has collaborated with leading professors and researchers in the field of cancer treatment, both in the United States and China.
These international experiences have broadened her perspective and contributed to her innovative approach to cancer therapy development.
Hau's work is particularly focused on addressing the challenges of treating advanced and metastatic cancers. She has been involved in clinical trials evaluating the safety and efficacy of various immunotherapy approaches, including the promising Gamma Delta T cell therapy.
- Comments Closed
- July 3rd, 2024




Advanced leukemia treatments, CAR-T clinical trials CLL, CAR-T for B-cell malignancies, CAR-T therapy for CLL, CD19-targeted CLL therapy, Chronic lymphocytic leukemia treatment, Immunotherapy for leukemia, Relapsed CLL options
CancerFax is the most trusted online platform dedicated to connecting individuals facing advanced-stage cancer with groundbreaking cell therapies.
Send your medical reports and get a free analysis.
🌟 Join us in the fight against cancer! 🌟
Привет,
CancerFax — это самая надежная онлайн-платформа, призванная предоставить людям, столкнувшимся с раком на поздних стадиях, доступ к революционным клеточным методам лечения.
Отправьте свои медицинские заключения и получите бесплатный анализ.
🌟 Присоединяйтесь к нам в борьбе с раком! 🌟