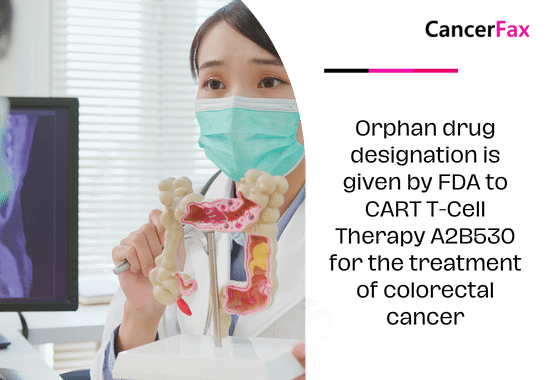
Orphan drug designation is given by FDA to CART T-Cell Therapy A2B530 for the treatment of colorectal cancer
In March 2024, a news release said that A2B530 (A2 Biotherapeutics), a CAR T-cell therapy, had been given Orphan Drug Designation to treat colorectal cancer that expresses carcinoembryonic antigen (CEA) and has lost HLA-A*02 expression in people who have germline heterozygous HLA-A*02(+) disease.
Researchers think that autologous logic-gated cell therapy can target tumor cells while protecting healthy tissue. This is because it has a built-in safety switch that can keep healthy tissue from getting hurt. In the phase 1/2 EVEREST-1 (NCT05736731) study, this idea will be tested.
You may like to read: CAR T-Cell therapy in China
The FDA’s decision to give Orphan Drug Designation confirms the huge unmet need for better treatments for people with colorectal cancer,” said William Go, MD, PhD, chief medical officer of A2 Bio, in a news release. This designation backs up our promise to use our cutting-edge technology platform to create new cancer treatments for people whose cancers are hard to treat.
The open-label, phase 1/2 EVEREST-1 study is looking at A2B530 as a possible treatment for solid tumors like colorectal cancer, pancreatic cancer, and non-small cell lung cancer. It also looks at other types of solid tumors that produce CEA but not HLA-A*02. The people who are now in the EVEREST-1 study were first in the BASECAMP-1 (NCT04981119) study, where their T cells were gathered, processed, and kept for later use.
You may like to read: CAR T-Cell therapy for multiple myeloma in China
The main goal of the phase 1 study is to find the safest and most effective dose. Safety and effectiveness must be maintained as the main goals of the phase 2 study. The amount of solid tumor cells that the recommended dose can kill while still saving healthy cells.
Colorectal cancer, which is a word for both colon and rectal cancer, happens when polyps (groups of cells growing together) form in the colon or rectum and turn into cancer. Older age, being black, having a history of polyps or cancer in your body or in your family, inflammatory bowel disease, genetics, diabetes, obesity, a normal Western diet, and smoking and drinking are all things that put people at risk.
Surgery, radiation, targeted therapy, and immunotherapy are the most common ways to treat colorectal cancer. However, many of the current methods for this cancer and others with solid tumors can kill patients.
You may like to read: CAR T Cell therapy cost in China
Researchers who worked on the study think that this CAR T-cell therapy is safer than other targeted treatments. This is because the CAR T-cells kill tumor cells without hurting healthy cells because they have a built-in safety switch that keeps healthy tissue from getting hurt.
Susan Hau is a distinguished researcher in the field of cancer cell therapy, with a particular focus on T cell-based approaches and cancer vaccines. Her work spans several innovative treatment modalities, including CAR T-cell therapy, TIL (Tumor-Infiltrating Lymphocyte) therapy, and NK (Natural Killer) cell therapy.
Hau's expertise lies in cancer cell biology, where she has made significant contributions to understanding the complex interactions between immune cells and tumors.
Her research aims to enhance the efficacy of immunotherapies by manipulating the tumor microenvironment and exploring novel ways to activate and direct immune responses against cancer cells.
Throughout her career, Hau has collaborated with leading professors and researchers in the field of cancer treatment, both in the United States and China.
These international experiences have broadened her perspective and contributed to her innovative approach to cancer therapy development.
Hau's work is particularly focused on addressing the challenges of treating advanced and metastatic cancers. She has been involved in clinical trials evaluating the safety and efficacy of various immunotherapy approaches, including the promising Gamma Delta T cell therapy.
- Comments Closed
- March 6th, 2024



A2B530 CAR-T orphan drug, Advanced colon cancer treatment, CAR-T for solid tumors, Colorectal cancer immunotherapy, CRC targeted therapy, FDA orphan designation 2024, HER2/CEA-targeted CAR-T, Rare cancer cell therapy
CancerFax is the most trusted online platform dedicated to connecting individuals facing advanced-stage cancer with groundbreaking cell therapies.
Send your medical reports and get a free analysis.
🌟 Join us in the fight against cancer! 🌟
Привет,
CancerFax — это самая надежная онлайн-платформа, призванная предоставить людям, столкнувшимся с раком на поздних стадиях, доступ к революционным клеточным методам лечения.
Отправьте свои медицинские заключения и получите бесплатный анализ.
🌟 Присоединяйтесь к нам в борьбе с раком! 🌟