Complete mechanism of CAR T Cell therapy explained
Introduction
CAR T cell therapy is a nascent cancer treatment that targets and eliminates cancerous cells by using the body’s immune system. Gene modification is done for the expression of chimeric antigen receptors on T cells, which identify and attach to a particular antigen on the surface of malignant cells. One needs to appreciate the underlying mechanisms of action of CAR T cell therapy if one is to understand its potential and how to surpass the current limitations.
Engineering CAR T Cells
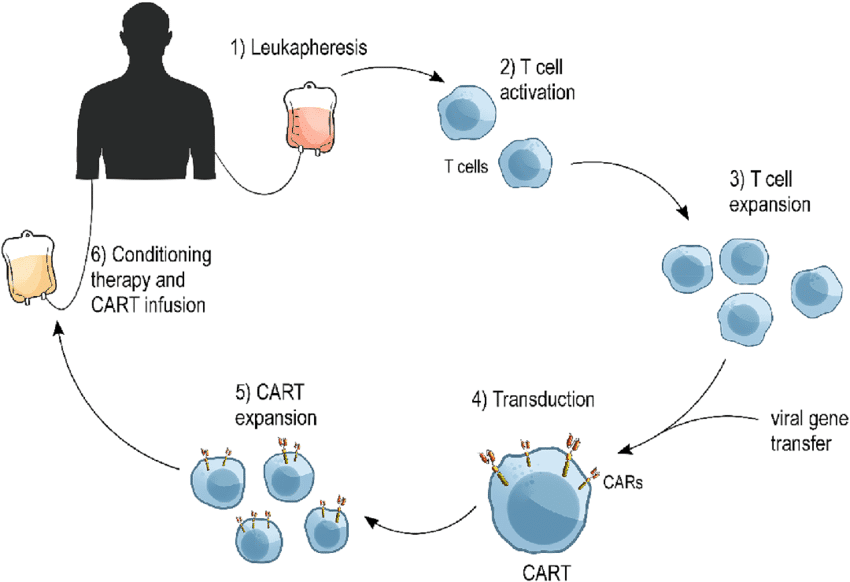
The production of CAR T cells begins with the isolation of T cells from a patient’s blood. After isolation, these T cells are genetically modified in a laboratory to express CARs. The CAR is a man-made receptor that has an antigen-binding domain and intracellular signaling domains that make T cells work and make more of themselves.
The structure of CAR has three components:
Antigen-Binding Domain: This domain typically comes from a monoclonal antibody that provides the CAR T cell with the ability to recognize and bind to specified antigens on the surface of a cancer cell.
Hinge and Transmembrane Domains: These parts connect the antigen-binding domain to the signaling domains inside the cell and hold the CAR to the T cell’s membrane.
Signaling Domains Inside Cells: These have activation motifs from the CD3ο chain and costimulatory molecules like CD28 or 4-1BB to help T cells become activated and stay activated more effectively.
Mechanism of Action
After being reinfused into the patient, the CAR T cells start circulating in the blood flow, looking for cancerous cells that express the target antigen, binding to them, and eventually activating the T cells. When the CAR molecule binds to its target antigen, it sets off a chain of events inside the cell that activate T-cells, cause them to multiply, and eventually release molecules that kill cells. Such released cytotoxic molecules would then induce programmed cell death in the targeted malignant cells.
Steps in the Mechanism of CAR-T Cell Therapy:
Recognition and Binding: The CAR T cell recognizes a certain antigen on the surface of the cancer cell and binds to that cell.
Activation: The binding event triggers intracellular signaling domains of the CAR, leading to T cell activation.
Proliferation: Activated CAR T cells proliferate to increase the number of effector cells available to attack cancer cells.
Cytotoxic Response: CAR T cells release cytotoxic granules containing perforin and granzymes that enter into the membrane of the cancer cell, inducing apoptosis.
Elimination of Cancer Cells: The induction of programmed cell death in the targeted, cancerous cells reduces the load of the tumor.
Efficacy and Challenges
The efficacy of CAR T cell therapy in treating certain hematologic malignancies is unprecedented, notably in B-cell acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Various clinical trials have produced a high complete remission rate among patients with relapsed or treatment-refractory disease.
Several challenges will, however, act to the detriment of this wide application:
Antigen escape: The tumor cells turn off or change the targeted antigen so that CAR T cells can not see it. This can happen through genetic mutation or epigenetic silencing.
Tumor microenvironment: The immunosuppressive tumor microenvironment can impair CAR T-cell activity and persistence.
Toxicity: Potentially severe adverse reactions involving CRS and neurotoxicity may appear, requiring serious management and close monitoring.
Persistence and exhaustion: CAR T cells are rendered exhausted, losing effector functions, thereby reducing long-term efficacy.
Strategies to Improve Efficacy
At present, researchers are working on various strategies to overcome these challenges and improve the efficacy of CAR T cell therapy. These strategies include:
1. Dual or tandem CARs: Generating CAR T cells to express two or more CARs against distinct antigens can prevent antigen escape.
2. Armored CARs: To oppose the hostile tumor microenvironment, CAR T cells can be engineered to produce cytokines or costimulatory ligands. Suicide genes or safety switches allow for selective elimination of CAR T cells in case of profound toxicity.
Reconstruction Optimized: Manufacturing efficiency and scaling can be optimized for making CAR T cells, making them accessible and affordable.
Conclusion
The mechanism of CAR T cell therapy is such that it illustrates one of the very effective ways of using the immune system to attack cancer. It is in this setting that, by engineering T cells to express chimeric antigen receptors, therapy can specifically target and eliminate cancer cells, giving hope to patients with otherwise untreatable malignancies. These continuous struggles have pushed ongoing research and innovative strategies toward the aim of making the field of CAR T cell therapy more efficient and safe and expanding it to a larger number of cancers. Next-generation treatments that will overcome current limitations and provide durable remissions for patients with cancer will require an understanding of the intricate mechanisms underlying CAR T-cell therapy.
Dr. Nishant Mittal is a highly accomplished researcher with over 13 years of experience in the fields of cardiovascular biology and cancer research. His career is marked by significant contributions to stem cell biology, developmental biology, and innovative research techniques.
Research Highlights
Dr. Mittal's research has focused on several key areas:
1) Cardiovascular Development and Regeneration: He studied coronary vessel development and regeneration using zebrafish models1.
2) Cancer Biology: At Dartmouth College, he developed zebrafish models for studying tumor heterogeneity and clonal evolution in pancreatic cancer.
3) Developmental Biology: His doctoral work at Keio University involved identifying and characterizing medaka fish mutants with cardiovascular defects.
4) Stem Cell Research: He investigated the effects of folic acid on mouse embryonic stem cells and worked on cryopreservation techniques for hematopoietic stem cells.
Publications and Presentations
Dr. Mittal has authored several peer-reviewed publications in reputable journals such as Scientific Reports, Cardiovascular Research, and Disease Models & Mechanisms1. He has also presented his research at numerous international conferences, including the Stanford-Weill Cornell Cardiovascular Research Symposium and the Weinstein Cardiovascular Development Conference.
In summary, Dr. Nishant Mittal is a dedicated and accomplished researcher with a strong track record in cardiovascular and cancer biology, demonstrating expertise in various model systems and a commitment to advancing scientific knowledge through innovative research approaches.
- Comments Closed
- July 30th, 2024




Cancer immunotherapy explained, CAR-T cell engineering, CAR-T cell mechanism, CAR-T scientific basis, CAR-T treatment steps, Chimeric antigen receptor function, How CAR-T therapy works, T-cell activation process
CancerFax is the most trusted online platform dedicated to connecting individuals facing advanced-stage cancer with groundbreaking cell therapies.
Send your medical reports and get a free analysis.
🌟 Join us in the fight against cancer! 🌟
Привет,
CancerFax — это самая надежная онлайн-платформа, призванная предоставить людям, столкнувшимся с раком на поздних стадиях, доступ к революционным клеточным методам лечения.
Отправьте свои медицинские заключения и получите бесплатный анализ.
🌟 Присоединяйтесь к нам в борьбе с раком! 🌟