
CAR T-Cell therapy for leukemia
Introduction CAR T cell therapy is the breakthrough treatment against various kinds of leukemias, particularly B-cell ALL. In this treatment, genetically modified T cells are specifically designed to target antigens on cancer cel..
CAR T-Cell therapy for Chronic Lymphocytic Leukemia
Introduction Chronic lymphocytic leukemia (CLL) is a long-lasting condition where mature B lymphocytes, which have encountered antigens before, become cancerous. This leads to the buildup of malignant B cells in the blood, bone m..

CAR - T Cell therapy, CAR T Therapy, CAR T-Cell, chimeric agent
A Deep Dive into CAR T Cell Therapy: How Does It Work?
Discover the science behind CAR T Cell therapy treatment in India! Explore how this revolutionary treatment transforms your immune cells into cancer fighters. Read our blog now to learn more about this miraculous therapy and how ..

CAR T-Cell, CAR therapy, Immunotherapy, T cell therapy
Is CAR T-Cell Therapy Available In India?
Have you ever wondered if there is a powerful way to fight cancer? Now just imagine if one day you found a ray of hope in your fight against cancer, a treatment that uses the power of your body's own immune system to target an..
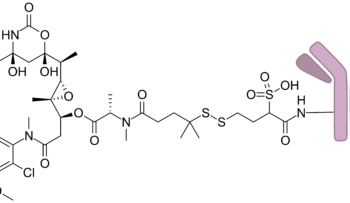
IDH1 mutation, olutasidenib, refractory acute myeloid leukemia, Rezlidhia
Olutasidenib is approved by FDA for relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation
Dec 2022: Olutasidenib (Rezlidhia) capsules were approved by the Food and Drug Administration (FDA) for adult patients with relapsed or resistant acute myeloid leukaemia (AML) who have a susceptible IDH1 mutation as identifie..
Asparaginase erwinia chrysanthemi, Jazz Pharmaceuticals, Rylaze
New dosing regimen for asparaginase erwinia chrysanthemi (recombinant) is approved by FDA
Dec 2022: A new Monday-Wednesday-Friday dosing schedule for asparaginase erwinia chrysanthemi (recombinant)-rywn has been approved by the Food and Drug Administration (Rylaze, Jazz Pharmaceuticals). Patients should receive 25 mg/m..
ivosidenib, NCT03173248, Servier Pharmaceuticals LLC, Tibsovo
Ivosidenib in combination with azacitidine is approved for newly diagnosed acute myeloid leukemia
June 2022: Ivosidenib (Tibsovo, Servier Pharmaceuticals LLC) in combination with azacitidine has been approved by the Food and Drug Administration for newly diagnosed acute myeloid leukaemia (AML) in adults 75 years or older with..
AZA-JMML-001, Celgene Corporation, juvenile myelomonocytic leukemia, NCT02447666, Vidaza
Azacitidine is approved by FDA for newly diagnosed juvenile myelomonocytic leukemia
June 2022: The FDA has approved the drug azacitidine (Vidaza, Celgene Corp.) for children with newly diagnosed juvenile myelomonocytic leukaemia (JMML).The pharmacokinetics, pharmacodynamics, safety, and activity of azacitidine p..
childhood leukemia, Chronic leukemias, induction chemotherapy, Leukaemia, paediatric leukaemia
Childhood leukemia and its treatment
Leukemia in childhood Leukemia is the most common cancer in children and teens, accounting for almost 1 out of 3 cancers. Most childhood leukemias are acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML). Chronic leu..
Burkitt lymphoma, Burkitt-like lymphoma, DLBCL, Genentech, Rituxan, Rituximab
Rituximab plus chemotherapy is approved by FDA for pediatric cancer indications
March 2022: The Food and Drug Administration has approved rituximab (Rituxan, Genentech, Inc.) in conjunction with chemotherapy for CD20-positive diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL), Burkitt-like lymphoma..