
CAR T-Cell Therapy for esophageal cancer
Esophageal cancer is a cancerous tumor that develops in the esophagus, the muscular tube through which food and liquids pass from the throat to the stomach. It is the sixth leading cause of cancer-related deaths across the globe.
While traditional treatments such as surgery, chemotherapy, and radiation therapy are still the mainstream approach, newer developments in immunotherapy have provided hopeful alternatives. One of these novel strategies is Chimeric Antigen Receptor (CAR) T Cell Therapy.
Esophageal Cancer
Esophageal cancer is a type of malignancy that occurs in the esophagus, the long, hollow tube that connects the throat to the stomach. It facilitates the movement of food and liquids from the mouth to the stomach for digestion. When cancer cells form in the inner lining of the esophagus, they can grow and invade deeper layers, potentially spreading to other parts of the body.
Types of Esophageal Cancer
There are two primary types of esophageal cancer:
Squamous Cell Carcinoma: This type develops in the squamous cells lining the upper and middle parts of the esophagus. It is more common in regions with high consumption of tobacco and alcohol.
Adenocarcinoma: This originates in the glandular cells responsible for mucus production, usually in the lower esophagus near the stomach. It is often linked to gastroesophageal reflux disease (GERD) and Barrett’s esophagus.
Stages of Esophageal Cancer
Esophageal cancer is staged based on the tumor size, lymph node involvement, and the presence of distant metastasis. The stages are:
Stage 0 (Carcinoma in situ): Abnormal cells are present in the innermost layer but haven’t spread.
Stage I: Cancer has formed and begun to grow into deeper layers of the esophagus.
Stage II: The cancer may have spread to nearby lymph nodes and further invaded the esophageal walls.
Stage III: Cancer has penetrated deeply into the esophagus and surrounding tissues, with significant lymph node involvement.
Stage IV: The cancer has metastasized to distant organs, such as the liver, lungs, or other parts of the body.
Early diagnosis and appropriate treatment, including surgery, chemotherapy, radiation therapy, or targeted therapies, can improve outcomes.
CAR T Cell therapy
It is one of the groundbreaking forms of immunotherapy that puts to bear the power of a person’s own immune system against cancer. This treatment involves making T cells carry a synthetic receptor known as CAR that specifically makes them recognize tumor-specific antigens expressed by cancer cells, and on infusing them back into the body, these engineered T cells can recognize and destroy cancer cells with remarkable precision.
CAR T-cell therapy has greatly succeeded in hematologic malignancies, including leukemia and lymphoma. The same cannot be said for its use in solid malignancies such as breast and lung cancer. Such is mainly attributed to heterogeneity of tumors, the immunosuppressive nature of the tumor microenvironment, and lack of targetable antigens. Many researchers are pursuing CAR T-cell therapy in the treatment of breast cancer with attention to HER2, MUC1, and FRα for specificity and potency.
Though promising, CAR T-cell therapy comes with potential side effects, including cytokine release syndrome (CRS) and neurotoxicity. Studies are underway to improve safety and efficacy, including the development of bispecific CARs and armored CAR T cells. As the clinical trials advance, CAR T-cell therapy promises to revolutionize cancer treatment and provide hope to patients with aggressive or treatment-resistant cancers.
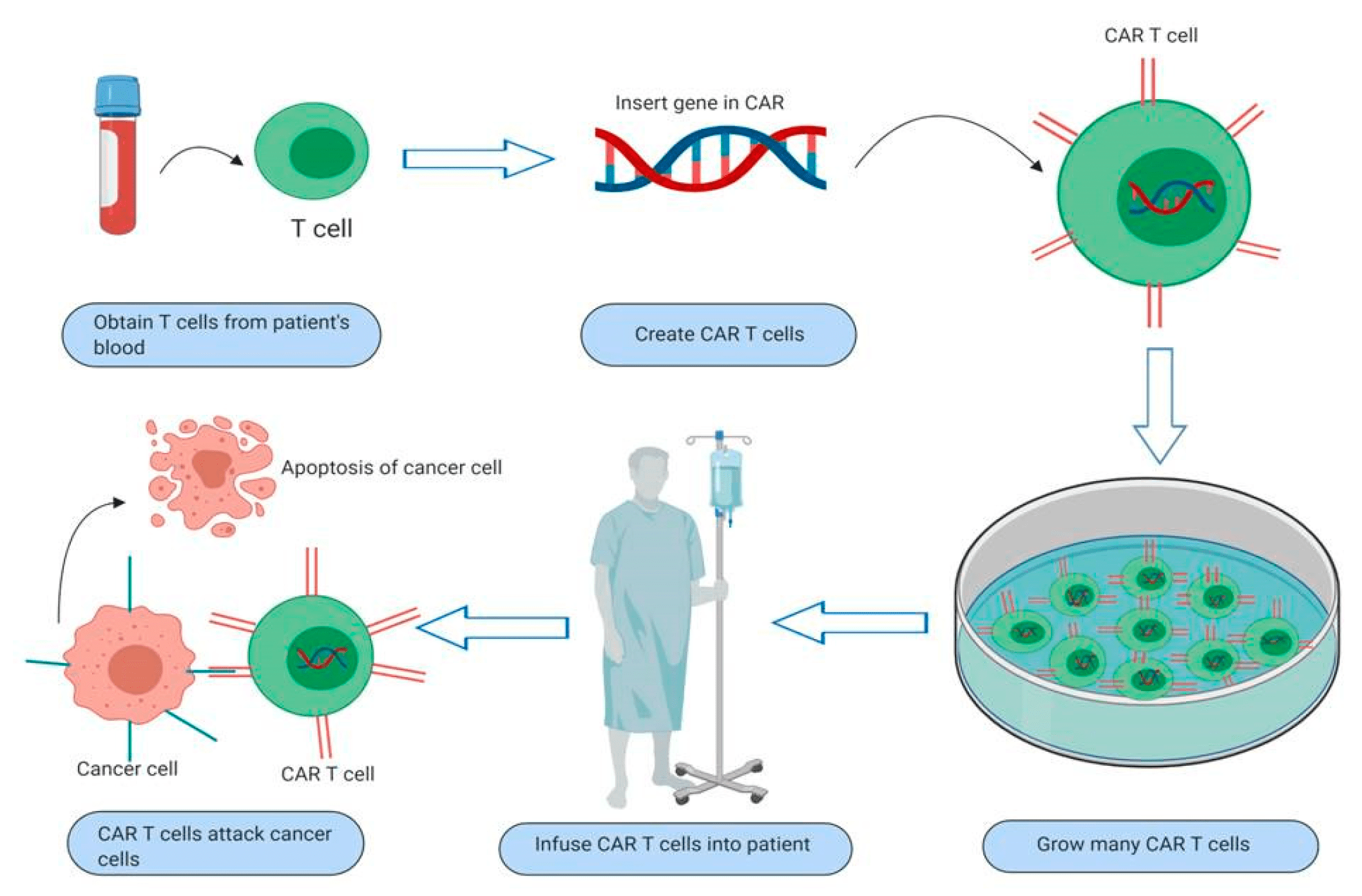
Pic: Working of CAR T Cell therapy
CAR T Cell Therapy in Esophageal Cancer
While CAR T therapy has shown remarkable success in hematologic malignancies like leukemia and lymphoma, its application in solid tumors like esophageal cancer has been challenging. However, ongoing research has made significant progress in overcoming these obstacles.
Some of the primary challenges in using CAR T cells for esophageal cancer include:
Tumor Microenvironment: Solid tumors create a protective environment that inhibits T cell infiltration and function.
Antigen Heterogeneity: Tumors may express a range of antigens, reducing the efficacy of single-target CAR T cells.
Toxicity: On-target, off-tumor effects can damage normal cells expressing the same antigens.
To address these issues, researchers are exploring multi-target CAR T cells, combination therapies, and armored CAR T cells equipped with cytokines that enhance immune response.
Key Target Antigens for Esophageal Cancer CAR T Therapy
Identifying the right target antigen is crucial for the success of CAR T therapy. Some promising target antigens in esophageal cancer include:
HER2 (Human Epidermal Growth Factor Receptor 2): Overexpressed in esophageal adenocarcinoma, HER2-targeted CAR T cells have shown promising preclinical results.
EGFR (Epidermal Growth Factor Receptor): Targeting EGFR with CAR T cells has demonstrated effectiveness in both esophageal squamous cell carcinoma and adenocarcinoma.
Claudin 18.2: This gastric cancer-associated antigen is also expressed in esophageal cancers, making it an attractive CAR T target.
MUC1: A glycoprotein commonly overexpressed in epithelial cancers, including esophageal cancer.
Recent Clinical Trials and Developments
China has emerged as a leader in CAR T cell research and development. With robust government support and an increasing number of biotech companies entering the space, China has made significant strides in bringing CAR T therapies to patients.
Innovative CAR T Designs: Chinese researchers are exploring dual-target CAR T cells that simultaneously target multiple antigens, improving treatment efficacy and reducing the risk of tumor escape.
Clinical Trial Expansion: China has approved numerous clinical trials investigating CAR T therapies for solid tumors, including esophageal cancer. Early-phase trials targeting HER2 and Claudin 18.2 have demonstrated encouraging results.
Cost-Effective Manufacturing: Chinese companies are developing streamlined manufacturing processes to reduce the overall cost of CAR T therapy, making it more accessible for a broader patient population.
Advancements in China
China has emerged as a leader in CAR T cell research and development. With robust government support and an increasing number of biotech companies entering the space, China has made significant strides in bringing CAR T therapies to patients.
Innovative CAR T Designs: Chinese researchers are exploring dual-target CAR T cells that simultaneously target multiple antigens, improving treatment efficacy and reducing the risk of tumor escape.
Clinical Trial Expansion: China has approved numerous clinical trials investigating CAR T therapies for solid tumors, including esophageal cancer. Early-phase trials targeting HER2 and Claudin 18.2 have demonstrated encouraging results.
Cost-Effective Manufacturing: Chinese companies are developing streamlined manufacturing processes to reduce the overall cost of CAR T therapy, making it more accessible for a broader patient population.
Side Effects and Management
While CAR T therapy holds immense potential, it can cause certain side effects, the most common being:
Cytokine Release Syndrome (CRS): A systemic inflammatory response that can lead to fever, low blood pressure, and organ dysfunction.
Neurotoxicity: Some patients may experience confusion, headaches, or seizures.
On-Target, Off-Tumor Toxicity: Damage to normal tissues expressing the same antigen targeted by CAR T cells.
Healthcare providers closely monitor patients during and after treatment to manage these side effects effectively using therapies such as tocilizumab and corticosteroids.
Conclusion
CAR T cell therapy is a revolutionary change in the therapy of cancer with a targeted, personalized solution to esophageal cancer patients. As research tries to overcome challenges with solid tumors, the promise of CAR T therapy for treating esophageal cancer in the future appears.
For cancer patients looking for a new way of advanced cancer treatment, CAR T cell therapy may bring new hope. Clinical trials ongoing and new innovations, especially in countries like China, are propelling developments to make the therapy more accessible and efficient.
Contact CancerFax for CAR T Cell Therapy
If you or your loved one is exploring CAR T cell therapy options for colorectal cancer or other solid tumors, CancerFax is here to help. Our team of experts can connect you with leading hospitals and clinics offering CAR T cell therapy.
Visit our website at www.cancerfax.com or contact us directly to learn more about eligibility, treatment options, and clinical trial participation.
Take the first step towards a personalized cancer treatment journey with CancerFax.