CAR T Cell therapy for AIDS related B-Cell malignancies
Introduction
CAR T cell therapy, an innovative form of immunotherapy, has demonstrated exceptional efficacy in the treatment of specific tumors by leveraging the potency of a patient’s own immune system. Recently, scientists have investigated the idea of using it to treat B cell malignancies associated with HIV, which is a complicated problem that involves both cancer and HIV/AIDS.
HIV patients have a far higher cancer risk than the normal population. HIV-positive people have 25%–40% cancer rates. HIV/AIDS patients’ most common malignancy is non-Hodgkin lymphoma (NHL). NHL is most common in PLWHA as Burkitt lymphoma, which has an 18-fold higher risk, and diffuse large B cell lymphoma (DLBCL), which accounts for 75% of cases.
Chimeric antigen receptor (CAR) T cell therapy, especially CD19-targeting CAR T cells, has revolutionized B cell NHL treatment. Due to safety concerns and the difficulties of generating CAR T cell products from HIV-positive donors, PLWHA has been excluded from all clinical studies for years. Recent studies investigated the use of HIV-positive lymphoma donors’ cells to create CD19-targeting CAR T cells. These studies have shown promise in addressing the safety and efficacy of CAR T cell treatment in this population, but they do not treat HIV.
In the past, CD19-CAR and N6-CAR T cells were effective in both clinical and preclinical testing against lymphoma and HIVgp120. It was anticipated that a dual design targeting both antigens may target cancer and HIV-infected cells in one person. A bi-specific N6-huCD19 CAR T cell technology was developed that targets both antigens in one therapeutic product.
Understanding the challenge
People who have HIV/AIDS have a higher chance of getting some types of cancer, especially B cell malignancies including lymphomas and leukemias. Treating these tumors in the presence of HIV might be challenging due to weakened immune function and difficulties in regulating immunosuppression.
How does CAR T therapy work?
CAR (chimeric antigen receptor) T cell treatment is the genetic modification of a patient’s T cells to express artificial receptors, called CARs, on their surface. These vehicles are engineered to identify particular substances found in malignant cells. Within the realm of HIV-related B cell malignancies, CAR T cells are specifically designed to recognize antigens such as CD19, which are frequently present on B cells.
Advantages and Challenges in HIV Patients
Utilizing CAR T cell treatment in HIV-positive individuals with B cell malignancies offers distinct benefits and obstacles. An advantage of CAR T cells is their high specificity in targeting cancer cells while preserving healthy cells, which can potentially reduce the risk of toxicities. Nevertheless, HIV infection poses challenges to immunological function, necessitating meticulous management of both the HIV virus and the malignancy.
Development of CAR-T for HIV
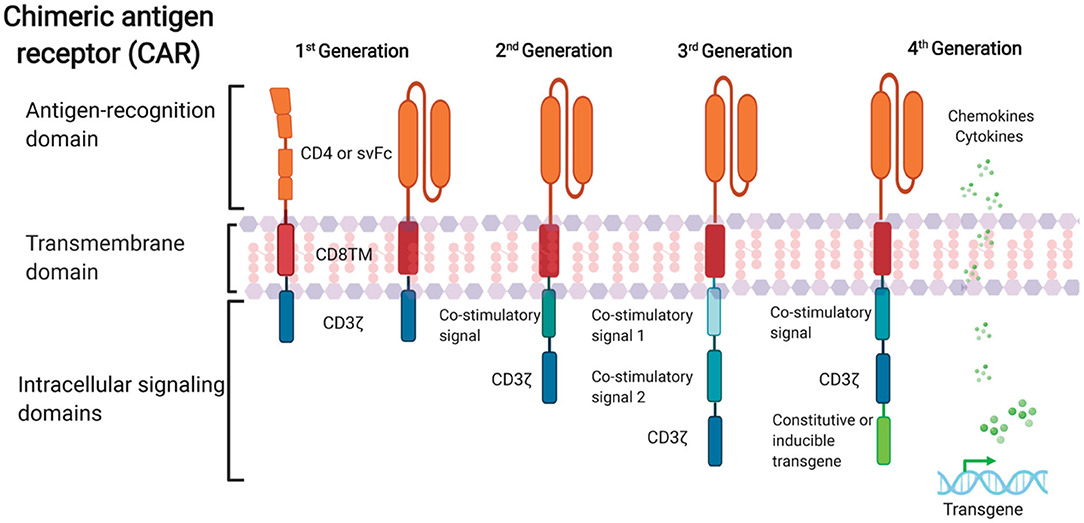
FIGURE 1. Schematic representation of anti-HIV CAR structure. First-generation CAR consists of a single anti-HIV env domain (CD4 or svFc), the transmembrane domain region, and the T cell receptor CD3-ζ domain. Second-generation CAR incorporates an additional costimulatory signaling domain into the basic first-generation receptor configuration. Third-generation cells contain more than one co-stimulatory domain. Fourth-generation CARs are characterized by the addition of constitutive or inducible transgenes like cytokines or chemokines.
Two tandem and one loop bi-specific CAR candidate designs were created. These constructs were created by integrating a humanized (hu) CD19 single-chain variable fragment (scFv) and an N6 scFv from an anti-HIV broadly neutralizing antibody (bNAb) into a 2nd-generation CAR construct backbone.
The bi-specific tandem CAR constructs had N6 and huCD19 scFvs coupled with a G4S linker in huCD19-N6 or N6-huCD19 orientation. The loop-CAR was created by fusing huCD19(VL):N6(VH):VL:VH with a whitlow linker. All constructs had the CD4 transmembrane domain, a double-mutated IgG4 Fc spacer, 4-1BB co-stimulatory and CD3-zetta signaling domains, and EGFRt separated by a T2A ribosomal skip region. The 3 bi-specific constructs were assessed in healthy donor-derived T cells employing cytotoxicity co-culture assay against Raji (CD19+) or 8E5 (gp120+) target cells. N6-huCD19 CAR T cells were produced from HIV-positive patients and tested against tumor cells.
Results
Functional testing showed that all three bi-specific CAR designs were effective against CD19 and HIVgp120. The N6-huCD19 tandem CAR T cells were more effective against both antigens. Notably, N6-huCD19 tandem CAR T cells may sequentially target one or both antigens.
N6-huCD19 tandem CAR T cells were generated from HIV-positive donors, proving this method is feasible in PLWHA. It is interesting that HIV donor-derived N6-huCD19 CAR T cells killed tumor cells that expressed either CD19 or HIVgp120 antigens, showing that they can do two different things.
You may like to read: CAR T Cell therapy in China
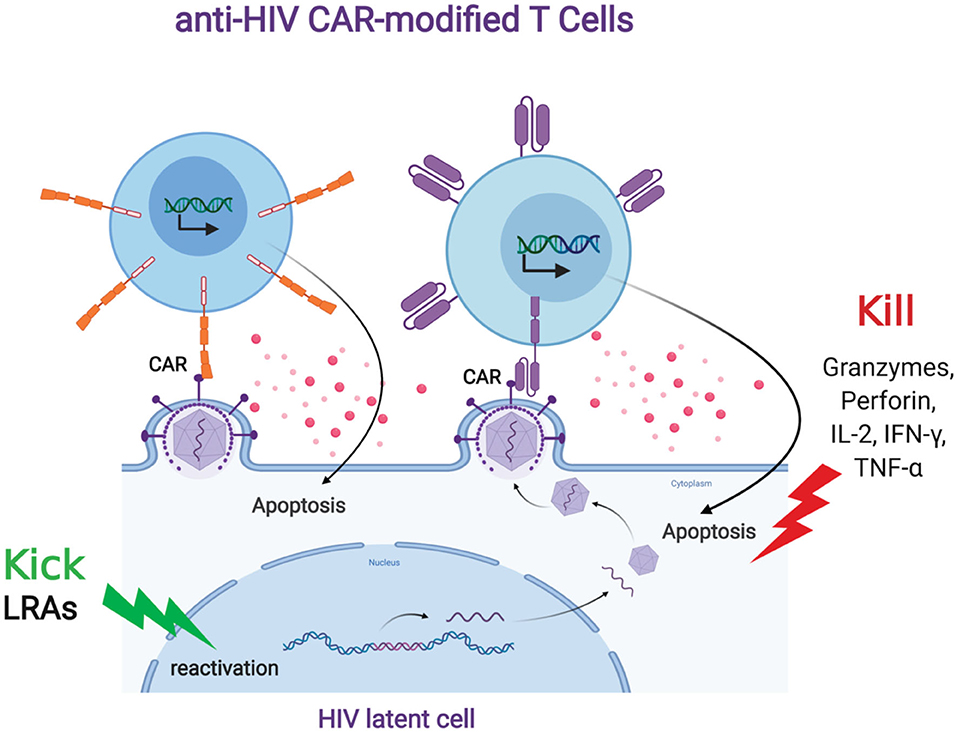
FIGURE 2. Anti-HIV CAR modified T cells and the “Kick and Kill” strategy to eliminate HIV latently infected cells. The “kick” strategy uses LRAs to induce HIV transcription, protein expression, and virion production. The CD4 (top left, orange) or antibody (top right, purple)-based CAR engineered T cell targets an HIV binding site on a viral particle on the cell surface of a reactivated reservoir cell (bottom). Upon binding, the CAR-modified T cell will release granzymes and cytokines to “kill” the HIV-infected cells.
Addressing Immunocompromise
An essential factor to address in CAR T cell therapy for HIV-related malignancies is the equilibrium between cancer treatment and HIV control. Individuals with weakened immune systems may be more prone to infections and may also face potential problems from immunotherapy treatment. Hence, it is imperative to closely evaluate and implement tailored treatment options.
CAR T Cells to Combat HIV Infection
The use of adoptive T cells to treat HIV was advocated decades ago. The first HIV CAR T cell therapy trial involved transplanting adoptive T cells with a CAR fusion of the CD4 extracellular domain (primary HIV receptor) to the CD3 signaling domain (CD4) (Mitsuyasu et al., 2000; Walker et al., 2000; Deeks et al., 2002). CD4, the natural HIV envelope recognition moiety, targets all HIV isolates, making it a good reactive ligand for anti-HIV CAR design. Mutations would reduce CD4 binding and viral fitness, hence, envelope protein CD4 binding sites are mostly conserved (Wang et al., 2019).
The first-generation CD4-based CAR was tested in HIV patients for efficacy and safety (Mitsuyasu et al., 2000; Walker et al., 2000; Deeks, 2002). The treatment did not inhibit viral replication, but the changed cells lasted for >10 years and had no overt toxicities (Mitsuyasu et al., 2000). The lack of viral control may have numerous causes: (1) CD4-based CARs make gene-modified T cells sensitive to HIV infection and cell death, (2) costimulatory signal activation signaling is inefficient, (3) T cell handling and multiplication are poor, and (4) viral antigen stimulation is lacking. However, CD4ζ T cell treatment was found to be safe and maintained stable engraftment levels (Scholler et al., 2012).
You may like to read: CAR T Cell therapy in India
Recent advances in CAR design to optimize CAR T cell effector function and persistence in malignancy have accelerated CAR T cell treatment. Four generations of CARs exist (Figure 1). The first generation of CARs connected an external antigen recognition moiety to a lymphocyte-stimulating intracellular endodomain, like the TCR CD3ζ chains’ signal-transducing component (Eshhar et al., 1993).
Apoptosis, limited in vivo growth, and cytotoxicity were common in first-generation CAR T cells (Heuser et al., 2003; Zhao et al., 2009). Second-generation CARs were created by adding costimulatory molecule domains like CD28 or 4-1BB to the cytoplasmic tail of CD3ζ-containing constructs. In vitro, optimized anti-HIV second-generation CAR T cells with the costimulatory 4-1BB domain suppressed HIV replication 50-fold better than first-generation CAR T cells (Leibman et al., 2017).
Animal experiments showed that secondary generation CARs expanded in response to antigen, protected CD4+T cells against HIV infection, and reduced CD4 decrease better than CARs without costimulatory molecules (Leibman et al., 2017). Comparable studies showed that the 4-1BB costimulatory domain reduces viral rebound after ART treatment and promotes T cell persistence in vivo without antigen better than the CD28 domain (Zhang et al., 2007; Leibman et al., 2017). Third-generation CARs were generated by adding numerous costimulatory molecules to secondary CARs. In cancer, third-generation CARs improve effector function, proliferation, survival, and tumor death (Savoldo et al., 2011).
Liu et al. (2016) found that a third-generation anti-gp120 CAR with numerous intracellular signaling domains (CD3ζ-CD28-41BB) has greater effectiveness in lysing Env-expressing cells in vitro than the CD4ζ-CAR. Fourth-generation CAR T cells, known as T cells redirected for universal cytokine-mediated killing (TRUCKs), contain a third stimulatory signal that secretes or tethers cytokines like IL-7, IL-12, IL-15, or IL-18 to improve CAR T cell expansion and persistence. They are being studied in oncology to target solid tumors.
Conclusion
CAR T cell therapy has great potential as an innovative treatment method for HIV-related B cell malignancies, providing new optimism for individuals dealing with this intricate disease overlap. Despite the existence of difficulties, continuous research and clinical endeavors are leading to the development of improved and tailored treatments that have the potential to revolutionize cancer care for those living with HIV.
Many efforts are underway to create new HIV eradication techniques. CAR-based techniques may help eradicate HIV by boosting the antiviral cellular immune response. A combination of techniques may be needed to successfully deploy anti-HIV CAR treatment to establish long-term immune surveillance and eliminate reactivated HIV cells. However, some questions remain for this field’s advancement:
(1) Can CAR-modified immune cells recognize LRA-induced reactivated antigen expression?
(2) Can CAR treatment and LRAs affect gut and brain tissue reservoirs?
(3) Are repeated CAR-modified immune cell infusions and/or LRA reactivations necessary?
Next-generation CAR T cell therapy as part of the “kick and kill” regimen, in combination with LRAs or bNAbs, may eliminate the HIV reservoir by improving immune surveillance and maintaining viral suppression after ART interruption. The best hospitals for CAR T cell therapy in China are conducting clinical trials in this area.
Susan Hau is a distinguished researcher in the field of cancer cell therapy, with a particular focus on T cell-based approaches and cancer vaccines. Her work spans several innovative treatment modalities, including CAR T-cell therapy, TIL (Tumor-Infiltrating Lymphocyte) therapy, and NK (Natural Killer) cell therapy.
Hau's expertise lies in cancer cell biology, where she has made significant contributions to understanding the complex interactions between immune cells and tumors.
Her research aims to enhance the efficacy of immunotherapies by manipulating the tumor microenvironment and exploring novel ways to activate and direct immune responses against cancer cells.
Throughout her career, Hau has collaborated with leading professors and researchers in the field of cancer treatment, both in the United States and China.
These international experiences have broadened her perspective and contributed to her innovative approach to cancer therapy development.
Hau's work is particularly focused on addressing the challenges of treating advanced and metastatic cancers. She has been involved in clinical trials evaluating the safety and efficacy of various immunotherapy approaches, including the promising Gamma Delta T cell therapy.
- Comments Closed
- April 18th, 2024





AIDS-related lymphoma CAR-T, B-cell cancers in HIV, CAR-T for immunocompromised, CAR-T safety in AIDS, CD19-targeted HIV oncology, Hematologic complications of HIV, HIV-associated malignancies, Precision immunotherapy for HIV+ patients
CancerFax is the most trusted online platform dedicated to connecting individuals facing advanced-stage cancer with groundbreaking cell therapies.
Send your medical reports and get a free analysis.
🌟 Join us in the fight against cancer! 🌟
Привет,
CancerFax — это самая надежная онлайн-платформа, призванная предоставить людям, столкнувшимся с раком на поздних стадиях, доступ к революционным клеточным методам лечения.
Отправьте свои медицинские заключения и получите бесплатный анализ.
🌟 Присоединяйтесь к нам в борьбе с раком! 🌟