Immuno-oncology is a way to treat cancer by using the body’s immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform that binds a patient’s own T cells to cancer cells. Because BiTE technology is flexible, it is easy to make molecules that attack tumor-specific antigens, which makes immuno-oncotherapy possible. Blinatumomab was the first standard BiTE molecule to be approved. It targets CD19 surface antigens on B cells and is mostly unaffected by genetic changes or escape mechanisms inside cells. More BiTE molecules are being made to treat other blood cancers (like multiple myeloma, acute myeloid leukaemia, and B-cell non-Hodgkin lymphoma) and solid tumours (like prostate cancer, glioblastoma, stomach cancer, and small-cell lung cancer). BiTE molecules that have a longer half-life than the standard ones are also being made. With BiTE technology, advances in immuno-oncology could make it easier to treat both blood and solid tumours and make them more effective when used with other treatments.
Ano ang BiTe therapy?
Immuno-oncology therapies are scientifically proven ways to treat different types of solid and mga cancer sa dugo. Hematologic cancers are a good fit for treatments that target the immune system because cancerous blood cells move around with immune cells. Several immunotherapy ang mga paggamot para sa kanser ay nasa mga gawa.
Monoclonal antibody checkpoint inhibitors that stop the binding of checkpoint proteins (like PD-1 and CTLA-4) are useful against many types of cancer. They work well and are safe for many solid tumours, especially when they target PD-1. Non-small-cell lung, kidney, and bladder cancers have all been treated successfully with these drugs. But many people don’t react to checkpoint inhibitors or get sick again after taking them. Except for non-Hodgkin lymphoma, most results on hematologic cancers have been disappointing, especially for myeloma and leukaemia, where the overall response rate in approved indications ranges from 12.0% to 48.5%.8-15.
Other immuno-oncology treatments, on the other hand, have a higher success rate. Chimeric antigen-receptor (CAR) T-cell therapies change a patient’s T cells to attack a specific cellular antigen, such as CD19 in the treatment of B-cell malignancies and B-cell maturation antigen (BCMA) in the treatment of maramihang myeloma (MM). CAR T-cell treatments have shown promise in treating hematologic cancers. They haven’t been as effective in treating solid tumours, but there have been some good results with neuroblastoma, human epidermal growth factor receptor tumours, and non-small-cell lung cancer. The genetic modification and in vitro multiplication of T cells take a long and complicated manufacturing process. This is a downside of this therapy because it makes it harder for patients to get this treatment quickly and in large numbers. The fact that lymphodepletion through chemotherapy preparation must be done first as a requirement for improved effectiveness is also a drawback.
Ang mga BiTE (bispecific T-cell engager) na mga therapies ay nag-uugnay sa sariling T cells ng pasyente sa mga tumor-expressed na antigens. Binubuksan nito ang cytotoxic na kakayahan ng sariling T cells ng pasyente na pumatay ng cancer nang hindi binabago ang mga gene ng T cells o nangangailangang palaguin o manipulahin ang mga ito sa labas ng katawan. Ang mga molekula ng BiTE ay maaaring gamitin nang mag-isa bilang mga gamot o kasama ng iba pang mga paggamot upang gawing mas epektibo ang mga ito.
BiTe mekanismo ng pagkilos
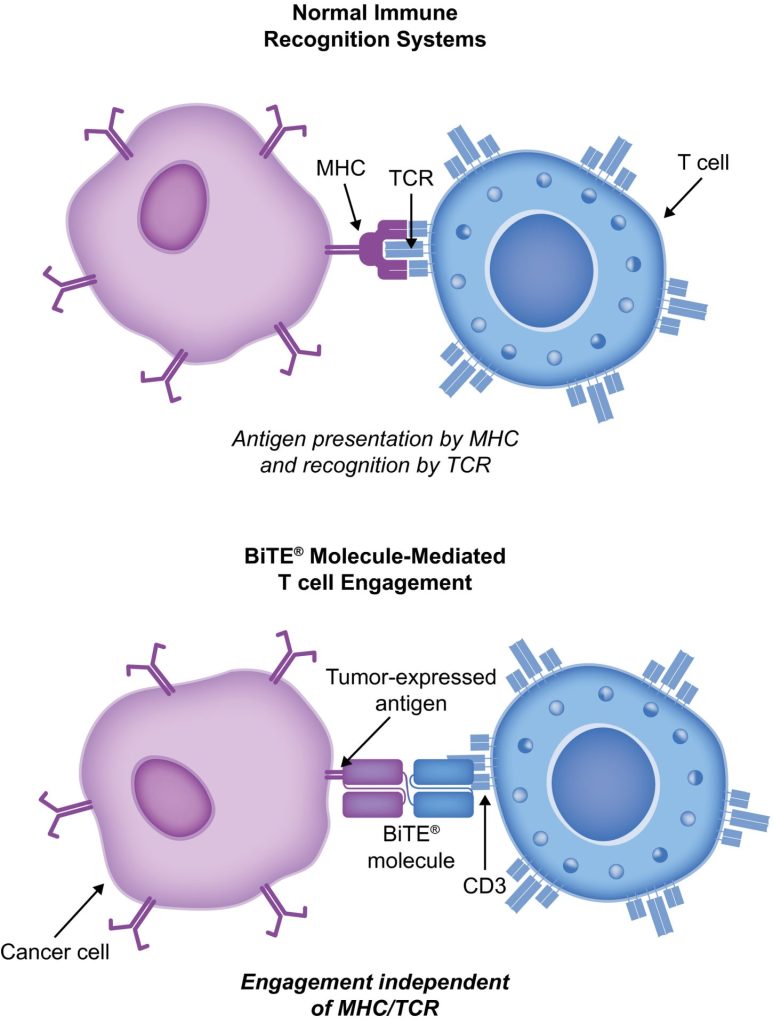
BiTE molecules are antibody constructs with two binding domains. One recognises tumor-expressed antigens (such as BCMA, CD19, or -like protein [DLL3]), and the other, CD3, recognises T cells (Fig. 1). Two single-chain variable fragment (scFv) regions from monoclonal antibodies are connected by a flexible peptide linker to make the binding domains. The first scFv binding region can be changed to target any surface antigen, so it can be used right away to treat a wide range of tumours and can be used again later. The second scFv binding region always binds to CD3, which is a part of the T-cell receptor complex that never changes. When a BiTE molecule interacts with both a cytotoxic T cell and a tumour cell, the T cells begin to multiply. This increases the amount of effector cells and makes BiTE therapy more effective. Then, the death of cancer cells is started. BiTE molecules can get any T cells to do this because they don’t need co-stimulation or the usual processes of the major histocompatibility complex.
Blinatumomab is the first and only BiTE therapy that has been approved. It targets the CD19 receptor on both normal and cancerous B cells. It is a highly potent molecule with cytotoxic effects seen at low exposures (10–100 pg/mL)26. In its presence, T cells can perform serial-target lysis, quickly binding to and killing many cells. This is how BiTE therapies work, and it can be seen in other BiTE molecules that are still in research. In talamak na lymphoblastic leukemia (LAHAT), blinatumomab has been shown to be effective and safe. In 2014, the US Food and Drug Administration gave it fast approval, and in 2017, it got full approval for relapsed or refractory (R/R) B-cell precursor (BCP) ALL. In 2018, accelerated approval was given to blinatumomab for treating BCP-ALL with minimum residual disease (MRD). This was the first approval for this use. In November 2015, the European Medicines Agency also gave it a green light for BCP-ALL with a Philadelphia chromosome (Ph) that is negative and R/R. Blinatumomab is approved for R/R BCP-ALL in adults and children in 57 countries, including Japan, all countries in the European Union, Canada, and Australia.
Blinatumomab para sa paggamot ng mga pasyente na may BCP-ALL
Binago ng Blinatumomab ang paraan ng pagtrato sa BCP-ALL. Kung ikukumpara sa standard-of-care (SOC) na chemotherapy, pinataas nito ang pangkalahatang kaligtasan (OS) at nabawasan ang bilang ng ilang mga side effect (AE). Ilang mahahalagang pag-aaral, kabilang ang mga randomized na kinokontrol na pagsubok, ay nagpakita na ang blinatumomab ay ligtas at gumagana para sa BCP-ALL sa parehong mga matatanda at bata. Therapy ng T-cell ng CAR, mayroon lamang data mula sa 2 single-arm na pag-aaral (clinicaltrials.gov IDs NCT01626495 at NCT01029366) kung saan 25 bata (edad 5–22) at 5 matanda (edad 26–60) na may R/R BCP-ALL at T-cell ALL ang ginamot. Ngunit ang mga resulta ay nangangako (isang kumpletong tugon [CR] sa 90%, napapanatiling remission na may 6 na buwang walang kaganapan na kaligtasan ng buhay sa 67%, at isang pangkalahatang rate ng kaligtasan [OS] na 78% [median follow-up, 7 buwan; saklaw, 1–24 na buwan]).
Ang TOWER study (A Phase 3, Randomized, Open Label Study Investigating the Efficacy of the BiTE Antibody Blinatumomab Versus Standard of Care Chemotherapy in Adult Subjects With Relapsed/Refractory B-Precursor ALL; clinicaltrials.gov identifier NCT02013167) kumpara sa mga epekto ng pre-blinatumomab na Pho-Cmotherapy na may Blinatumomab na pang-adulto na chemotherapy, R. CP-LAHAT. Dahil mas matagal ang buhay ng mga tao, maagang natigil ang pag-aaral. Ang mga AE sa pangkat na blinatumomab ay kapareho ng mga nakita sa mga naunang pag-aaral, at ang blinatumomab ay may mas mababang mga rate ng AE na nababagay sa pagkakalantad kaysa sa SOC.34 Gumagana rin ang Blinatumomab para sa mga taong may Ph-positive, R/R BCP-ALL at para sa mga batang may Ph-negative, R/R BCP-ALL.
30% to 50% of people with BCP-ALL in complete hematologic remission show persistent MRD. In the single-arm, phase 2 BLAST study (A Confirmatory Multicenter, Single-Arm Study to Assess the Efficacy, Safety, and Tolerability of the BiTE Antibody Blinatumomab in Adult Patients With MRD of B-Precursor Acute Lymphoblastic Leukaemia; clinicaltrials.gov identifier NCT01207388), blinatumomab was tested on patients with BCP-ALL in first or later complete After blinatumomab treatment, 78% of patients who were MRD positive became MRD negative. The 5-year OS study showed a median OS of 36.5 months, and more than half of those who had a complete MRD response after the first cycle of blinatumomab were still alive at 5 years, which suggests that the treatment might be able to cure some patients. AEs were seen that were linked to cytokine release syndrome (CRS).31 Other studies, like NCT03023878 and NCT03340766, are still looking at blinatumomab in first-line settings and in combination with other treatments.
CD19-targeted treatments have been linked to failure because of the loss of CD19 antigen after treatment. The failure rates for blinatumomab range from 8% to 35%, and for CAR T-cell na mga therapy, they range from 39% to 65%.36-40 We don’t fully understand what causes therapy to fail, but one possibility is immunoediting, in which antigen loss is caused by a T-cell-dependent process called immunoselection, which lets tumour cells get away.41 Lineage switch and epitope loss under therapy pressure have also been suggested as ways for tumours to escape treatment. However, a recent study on epitope loss found that some CD19 isoforms that help CAR T-cells escape were already present at the time of diagnosis. This suggests that combining treatments might be helpful. Another thing that can cause immunotherapy to fail is called “inhibitory T-cell signalling.” In this case, the blocking programmed death ligand-1 (PD-L1) is interesting because it is more common in B-cell ALL cells from patients who don’t respond to blinatumomab and can make CD3 BiTE molecules less effective.43 By making a CD28/PD-L1 BiTE that triggers the CD28 co-stimulatory signal instead of the inhibitory signaling pathway that is usually seen when a T cell binds to a PD-L1-expressing cancer cell, this inhibition could be turned off.43 Dual-targeted CAR T cells are also being looked into as a way to make up for the loss of tumour antigens. This can be done by modifying each T cell with 2 CAR molecules and 2 different binding domains (dual-signaling CAR) or by putting 2 different binding domains on 1 CAR molecule at the same time (TanCAR).
Mga masamang kaganapan sa BiTE at sa pamamahala nito
Sa mga klinikal na pag-aaral ng blinatumomab, ang pinakakaraniwang AE ay lagnat, mababang bilang ng puting dugo, at mababang bilang ng platelet. Ang ilan sa pinakamahalagang panganib ay ang CRS, neurotoxicity, at mga pagkakamali sa droga. Ang neurotoxicity ay maaari ding mangyari sa CD19-specific na CAR T-cell na paggamot, ngunit maaaring hindi ito dahil sa CD19. Ang mga resulta ng isang phase 1/1b na pag-aaral na nagpapatuloy pa rin tungkol sa mga target na CD20/CD3 ay nagpakita na ang mga CNS AE ng grade 3 o mas mataas ay bihira (3% ng lahat ng grade 3 AE). Kadalasan, ang reaksyon ng blinatumomab sa CRS ay banayad, ngunit sa mga bihirang kaso, maaari itong maging malubha at kahit na nagbabanta sa buhay. Ang mga nagpapasiklab na reaksyon ay maaaring mabawasan sa pamamagitan ng corticosteroids. Upang mapababa ang pagkakataon ng CRS, pinakamahusay na magbigay ng pagbubuhos ng prednisone o dexamethasone bago ang unang dosis ng blinatumomab at dahan-dahang taasan ang dosis. Ang paggamit na ito ng corticosteroids bago ang iba pang mga molekula ng BiTE ay nagbigay ng dahilan upang gamitin ang dexamethasone bilang isang premedication kapag gumagamit ng iba pang mga molekula ng BiTE. Gayunpaman, hindi malinaw kung ang epektong ito ay maaaring ilapat sa buong platform ng BiTE, at ang iba pang mga paraan upang harapin ang CRS ay tinitingnan. Ang Interleukin 6 ay isang cytokine na nagdudulot ng CRS at mataas sa mga taong mayroon nito. Ang Tocilizumab, na humaharang sa interleukin-6 receptor, ay ginamit upang gamutin ang CRS na napakasama pagkatapos ng paggamot sa CAR T-cell.49 Sa ospital, ang mga tumor necrosis factor-inhibitor ay ginamit din upang gamutin ang CRS.


