Glioblastoma Multiforme
About Disease
Glioblastoma is an aggressive form of cancer that can develop in the brain or spinal cord. Astrocytes, which support nerve cells, give rise to glioblastoma.
Although glioblastoma can develop at any age, it often affects older individuals more frequently. Seizures, nausea, vomiting, and increasing headaches are all possible effects. It can be very challenging to treat glioblastoma, also known as glioblastoma multiforme, and a cure is frequently not achievable. Cancer treatments may lessen symptoms and delay the disease’s progression.
Glioblastoma (GBM), commonly known as a grade IV astrocytoma, is an aggressive brain tumor that grows quickly. However, it typically does not migrate to distant organs, rather invading the nearby brain tissue.
GBMs can develop from lower-grade astrocytomas or develop from new brain tumors. The cerebral hemispheres of adults, particularly the frontal and temporal lobes, are where GBM develops most frequently. It is crucial to seek specialist neuro-oncological and neurosurgical care right away because GBM is a deadly brain cancer that, if left untreated, can cause death in six months or less. Such treatment can affect overall survival.
GBMs present unique treatment challenges due to:
- Localization of tumors in the brain
- Inherent resistance to conventional therapy
- Limited capacity of the brain to repair itself
- Migration of malignant cells into adjacent brain tissue
- The variably disrupted tumor blood supply, which inhibits effective drug delivery
- Tumor capillary leakage, resulting in an accumulation of fluid around the tumor, (peritumoral edema) and intracranial hypertension
- Tumor-induced seizures
- The resultant neurotoxicity of treatments directed at gliomas
Overview
Causes
Symptoms
Symptoms vary depending on the location of the brain tumor but may include any of the following:
- Persistent headaches
- Double or blurred vision
- Vomiting
- Loss of appetite
- Changes in mood and personality
- Changes in ability to think and learn
- New onset of seizures
- Speech difficulty of gradual onset
Diagnosis
Diagnosis of glioblastoma is done by using the following procedures:
Neurological examination: Your doctor will inquire about your signs and symptoms while performing a neurological exam. Your eyesight, hearing, balance, coordination, strength, and reflexes might all be tested. Problems in one or more of these regions could reveal information about the area of your brain that a brain tumor might impact.
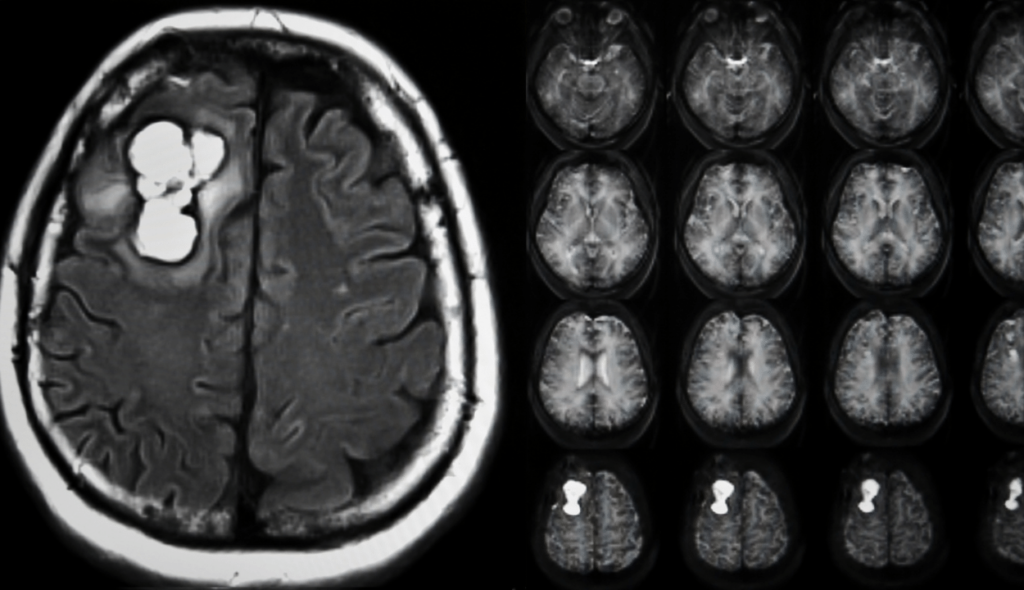
Imaging tests: Your doctor can assess the location and size of your brain tumor with the aid of imaging tests. Brain tumors are frequently diagnosed with MRI, which may also be combined with specific MRI imaging techniques like functional MRI and magnetic resonance spectroscopy.
CT and positron emission tomography are examples of further imaging tests (PET).
Biopsy: Depending on your unique circumstances and the location of your tumor, a needle biopsy may be performed either before or during surgery to remove your glioblastoma. The types of cells and their level of aggressiveness are identified by laboratory analysis of the sample of questionable tissue.
Your doctor can find out the types of mutations the tumor cells have acquired through specialized tests. This knowledge provides your doctor with hints regarding your prognosis and could influence the course of your therapy.
Grading of glioblastoma
After a brain tumor is detected on a CT or MRI scan, a neurosurgeon obtains tumor tissue for a biopsy, and the tissue is examined by a neuropathologist.
The analysis of tumor tissue is used to assign the tumor a name and grade and to provide answers to the following questions:
- What is the type of tumor, and how is it classified based on the WHO tumor classification?
- Are there signs of rapid growth in the tumor cells? What is the tumor grade?
| Histologic Grading | |
|---|---|
| GRADE II | Cytologic atypia (variation in nuclear shape and size + hyperchromasia) |
| GRADE III | Anaplasia and increased mitotic activity (increased cellularity) |
| GRADE IV | Microvascular proliferation and necrosis |
- Are there any specific genetic mutations within the tumor that can help with prognosis, help predict response to therapy, and assess the presence of experimental therapeutic targets?
Next-generation sequencing aids molecular analysis and profiling brain tumors to improve diagnostic accuracy, therapeutic target identification, and prognosis prediction. A few important alterations are in the table below.
| Important Molecular Alterations in Glioblastoma | |
|---|---|
| IDH mutation | Prognostic value, potential therapeutic target |
| MGMT methylation status | Prognostic value, predictive value for response to temozolomide |
| EGFR mutation | Diagnostic marker for glioblastoma, potential therapeutic target |
| TERT promoter mutation | Diagnostic marker for glioblastoma |
| Gain for 7p and loss of 10q | Diagnostic marker for glioblastoma |
| H3F3A | Diagnostic marker for a subset of gliomas (H3 K27M-mutant and H3 G34 mutant), therapeutic target |
| FGFR fusion | Therapeutic target |
| NTRK fusion | Therapeutic target |
Treatment and Management
Surgery is the cornerstone of GBM treatment, followed by radiation and chemotherapy. Surgery’s main goal is to cut off as much of the tumor as possible without harming the healthy brain tissue that surrounds it and is necessary for regular neurological function. It is hard to ever completely eradicate a GBM because the tumor is surrounded by a zone of moving, invading tumor cells that attack nearby tissues.
Surgery can lessen the amount of solid tumor tissue in the brain, remove tumor cells that may be chemotherapy- and radiation-resistant at the tumor’s centre, and lower intracranial pressure. Surgery has the potential to extend the lives of some patients and enhance the quality of their remaining years by removing the mass of the tumor.
The majority of the time, a craniotomy is used by surgeons to access the tumor site. The locations of the motor, sensory, and speech/language cortex are frequently determined using computer-assisted image guidance and occasionally employing intraoperative mapping techniques. Intraoperative mapping frequently entails doing surgery on an awake patient while mapping the anatomy of their language function. The physician next determines which areas of the tumor can be safely removed.
Radiation therapy can start after surgery, once the wound has healed. Radiation therapy aims to specifically eradicate any leftover tumor cells that have spread into the surrounding healthy brain tissue. Standard external beam radiation therapy involves repeated sessions of standard-dose radiation “fractions” being administered to the tumor site and a margin to target the tumor cells that have infiltrated there. Both healthy and normal tissue are damaged by each treatment.
Most of the normal cells have healed the damage by the time the subsequent therapy is administered, but the tumor tissue has not. Depending on the type of tumor, this process is repeated for a total of 10 to 30 treatments, which are typically administered once per day, five days a week. Compared to surgery alone or the greatest supportive care, radiation therapy offers the majority of patients better outcomes and longer survival rates.
Radiosurgery is a type of medical procedure that concentrates radiation on the tumor while delivering the least amount of radiation possible to the surrounding brain tissue. In some instances, radiosurgery may be utilized to treat tumor recurrence, frequently with the use of additional data from MRS or PET scans. In the early stages of GBM treatment, it is rarely employed.
Special medications that are intended to kill tumor cells are given to patients receiving chemotherapy. The current gold standard of care for GBM involves chemotherapy with the medication temozolomide. During radiation therapy, the medication is often given every day, and then for six cycles after radiation, during the maintenance phase.
The first five days of each cycle are spent administering temozolomide, followed by 23 days of rest. Each cycle lasts for 28 days. During the maintenance stage of treatment, tumor treating fields are introduced as a different type of treatment. Alternating electrical fields are produced, which stop cancer cells from proliferating and dividing. When the tumor grows, mostly lomustine (chemotherapy) and bevacizumab (targeted therapy) are utilized.
CAR T-Cell therapy for glioblastoma multiforme
A recently created immunotherapy for the treatment of tumors is called chimeric antigen receptor-engineered T-cell (CAR-T) therapy. Its use has been investigated in the treatment of solid tumors, such as glioblastoma multiforme, because CAR-T therapy in China has shown remarkable efficacy in treating CD19-positive hematological malignancies.