
Capivasertib with fulvestrant is approved by the USFDA for breast cancer treatment
Capivasertib (Truqap, AstraZeneca Pharmaceuticals) in combination with fulvestrant was approved by the Food and Drug Administration on November 16, 2023, for adult patients with hormone receptor (HR)-positive, human epidermal gro..

The Inside Scoop on Breast Cancer Treatment Costs in India – A Must-Read Revelation!
Breast cancer accounts for 31% of all cancers diagnosed in Indian women, making it the leading type of cancer. This serious disease must be treated at an early stage for the best outcomes. Our blog breaks down the breast cancer t..

Abemaciclib, aromatase inhibitor, Eli Lilly and Company, endocrine therapy, Tamoxifen, Verzenio
Abemaciclib with endocrine therapy is approved by FDA in HER 2 positive breast cancer
March 2023: Abemaciclib (Verzenio, Eli Lilly and Company) and endocrine therapy (tamoxifen or an aromatase inhibitor) have been approved by the Food and Drug Administration (FDA) for the adjuvant treatment of adult patients with ..

China, Enhertu, HER 2 positive
Enhertu has been approved in China for patients with HER2-positive metastatic breast cancer
Feb 2023: Enhertu (trastuzumab deruxtecan) from AstraZeneca and Daiichi Sankyo has been approved as a monotherapy for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received ..

Hamsa, Hamsa Nandini, Mirchi, Mirchi movie
Actor Hamsa Nandini Provides an Update One Year After Breast Cancer Treatment
Feb 2023: Hamsa Nandini, who was diagnosed with Grade III Invasive Carcinoma (Breast Cancer) in 2021, has updated her Instagram followers on her health status. The actress, who has appeared in Telugu films such as Mirchi and Lege..
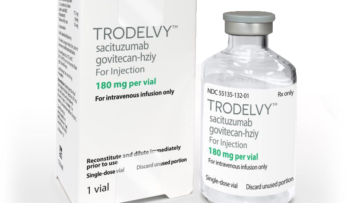
Gilead Sciences, sacituzumab govitecan-hziy, Trodelvy, TROPiCS-02
Sacituzumab govitecan-hziy is approved by FDA for HR-positive breast cancer
Feb 2023: The Food and Drug Administration (FDA) has approved sacituzumab govitecan-hziy (Trodelvy, Gilead Sciences, Inc.) for people with hormone receptor (HR)-positive, HER2-negative (IHC 0, IHC 1+, or IHC 2+/ISH-) breast cance..
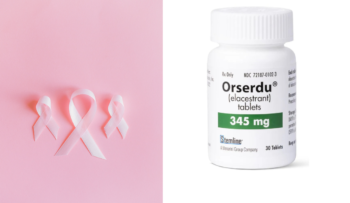
elacestrant, Orserdu, Stemline Therapeutics
Elacestrant is approved by FDA for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer
In February 2023, the Food and Drug Administration (FDA) approved elacestrant (Orserdu, Stemline Therapeutics, Inc.) for women or men over 50 who have advanced or metastatic breast cancer and are ER-positive, HER2-negative, and h..
Daiichi Sankyo, Enhertu, fam-trastuzumab deruxtecan-nxki
Fam-trastuzumab deruxtecan-nxki is approved by FDA for breast cancer
April 2022: Adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting and have develop..
AstraZeneca Pharmaceuticals, BRCA, gBRCAm, Lynparza, olaparib, OlympiA
Olaparib is approved for adjuvant treatment of high-risk early breast cancer
March 2022: The Food and Drug Administration has approved olaparib (Lynparza, AstraZeneca Pharmaceuticals, LP) for the adjuvant treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) h..
Abemaciclib, Agilent, Dako Omnis, Eli Lilly and Company, endocrine therapy, Tamoxifen, Verzenio
Abemaciclib is approved by FDA with endocrine therapy for early breast cancer
October 2021: The Food and Drug Administration has approved abemaciclib (Verzenio, Eli Lilly and Company) in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) for adjuvant treatment of adult patients with ho..