Immuno-oncology is a way to treat cancer by using the body’s immune system. BiTE (bispecific T-cell engager) technology is a targeted immuno-oncology platform that binds a patient’s own T cells to cancer cells. Because BiTE technology is flexible, it is easy to make molecules that attack tumor-specific antigens, which makes immuno-oncotherapy possible. Blinatumomab was the first standard BiTE molecule to be approved. It targets CD19 surface antigens on B cells and is mostly unaffected by genetic changes or escape mechanisms inside cells. More BiTE molecules are being made to treat other blood cancers (like multiple myeloma, acute myeloid leukaemia, and B-cell lymphoma neamh-Hodgkin) and solid tumours (like prostate cancer, glioblastoma, stomach cancer, and small-cell lung cancer). BiTE molecules that have a longer half-life than the standard ones are also being made. With BiTE technology, advances in immuno-oncology could make it easier to treat both blood and solid tumours and make them more effective when used with other treatments.
Cad is teiripe BiTe ann?
Immuno-oncology therapies are scientifically proven ways to treat different types of solid and ailsí fola. Hematologic cancers are a good fit for treatments that target the immune system because cancerous blood cells move around with immune cells. Several immunotherapy cóireálacha le haghaidh ailse i láthair na huaire.
Monoclonal antibody checkpoint inhibitors that stop the binding of checkpoint proteins (like PD-1 and CTLA-4) are useful against many types of cancer. They work well and are safe for many solid tumours, especially when they target PD-1. Non-small-cell lung, kidney, and bladder cancers have all been treated successfully with these drugs. But many people don’t react to checkpoint inhibitors or get sick again after taking them. Except for non-Hodgkin liomfóma, most results on hematologic cancers have been disappointing, especially for myeloma and leukaemia, where the overall response rate in approved indications ranges from 12.0% to 48.5%.8-15.
Other immuno-oncology treatments, on the other hand, have a higher success rate. Chimeric antigen-receptor (CAR) T-cell therapies change a patient’s T cells to attack a specific cellular antigen, such as CD19 in the treatment of B-cell malignancies and B-cell maturation antigen (BCMA) in the treatment of il-mhóilíní (MM). CAR T-cell treatments have shown promise in treating hematologic cancers. They haven’t been as effective in treating solid tumours, but there have been some good results with neuroblastoma, human epidermal growth factor receptor tumours, and non-small-cell lung cancer. The genetic modification and in vitro multiplication of T cells take a long and complicated manufacturing process. This is a downside of this therapy because it makes it harder for patients to get this treatment quickly and in large numbers. The fact that lymphodepletion through chemotherapy preparation must be done first as a requirement for improved effectiveness is also a drawback.
Nascann teiripí BiTE (gealltóir T-chealla déspecálach) cealla T an othair féin le hantaiginí siadaí-léirithe. Casann sé seo ar chumas citotocsaineach cealla T an othair féin ailse a mharú gan géinte na gcealla T a athrú nó gan iad a fhás nó a ionramháil lasmuigh den chorp. Is féidir móilíní BitTE a úsáid ina n-aonar mar chógais nó le cóireálacha eile chun iad a dhéanamh níos éifeachtaí.
Meicníocht gníomhaíochta bite
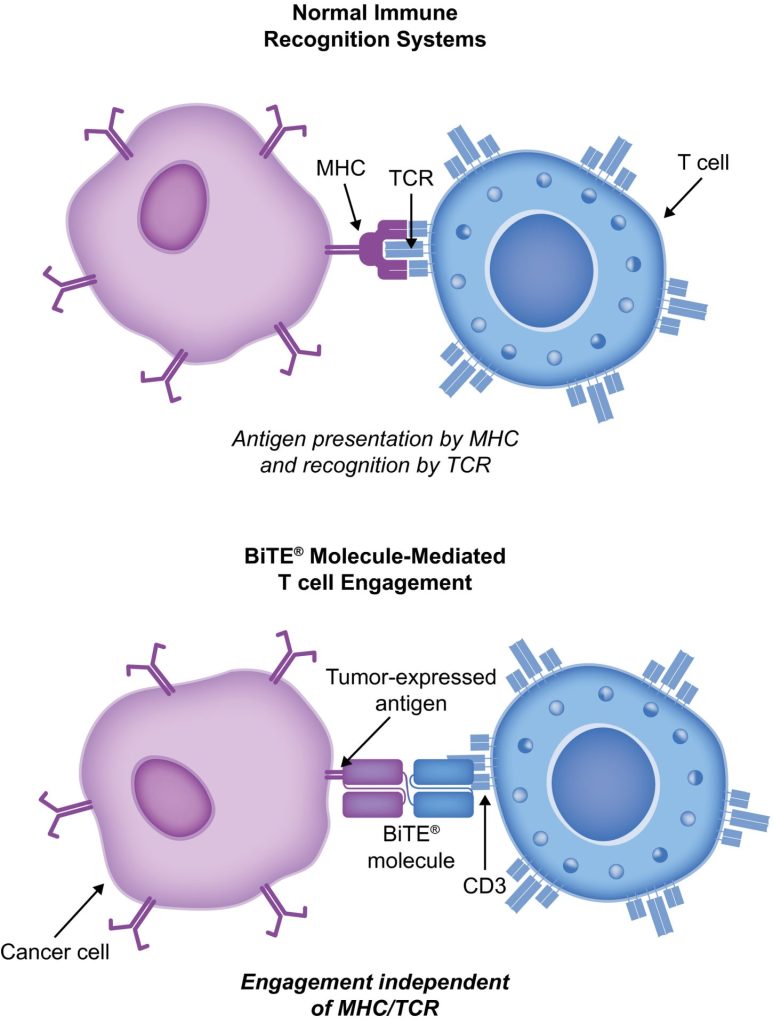
BiTE molecules are antibody constructs with two binding domains. One recognises tumor-expressed antigens (such as BCMA, CD19, or -like protein [DLL3]), and the other, CD3, recognises T cells (Fig. 1). Two single-chain variable fragment (scFv) regions from monoclonal antibodies are connected by a flexible peptide linker to make the binding domains. The first scFv binding region can be changed to target any surface antigen, so it can be used right away to treat a wide range of tumours and can be used again later. The second scFv binding region always binds to CD3, which is a part of the T-cell receptor complex that never changes. When a BiTE molecule interacts with both a cytotoxic T cell and a tumour cell, the T cells begin to multiply. This increases the amount of effector cells and makes BiTE therapy more effective. Then, the death of cancer cells is started. BiTE molecules can get any T cells to do this because they don’t need co-stimulation or the usual processes of the major histocompatibility complex.
Blinatumomab is the first and only BiTE therapy that has been approved. It targets the CD19 receptor on both normal and cancerous B cells. It is a highly potent molecule with cytotoxic effects seen at low exposures (10–100 pg/mL)26. In its presence, T cells can perform serial-target lysis, quickly binding to and killing many cells. This is how BiTE therapies work, and it can be seen in other BiTE molecules that are still in research. In leoicéime lymphoblastic géarmhíochaine (GACH), blinatumomab has been shown to be effective and safe. In 2014, the US Food and Drug Administration gave it fast approval, and in 2017, it got full approval for relapsed or refractory (R/R) B-cell precursor (BCP) ALL. In 2018, accelerated approval was given to blinatumomab for treating BCP-ALL with minimum residual disease (MRD). This was the first approval for this use. In November 2015, the European Medicines Agency also gave it a green light for BCP-ALL with a Philadelphia chromosome (Ph) that is negative and R/R. Blinatumomab is approved for R/R BCP-ALL in adults and children in 57 countries, including Japan, all countries in the European Union, Canada, and Australia.
Blinatumomab le haghaidh cóireáil othar le BCP-ALL
D'athraigh Blinatumomab an bealach a gcaitear le BCP-ALL. I gcomparáid le ceimiteiripe caighdeánach cúraim (SOC), tá méadú tagtha ar mharthanas foriomlán (OS) agus laghdaigh líon na bhfo-iarmhairtí áirithe (AEanna). Léirigh roinnt staidéar tábhachtach, lena n-áirítear trialacha randamacha rialaithe, go bhfuil blinatumomab sábháilte agus go n-oibríonn sé do BCP-ALL i ndaoine fásta agus leanaí araon. Teiripe CAR T-chill, níl ach sonraí ó 2 staidéar aon-láimhe (clinicaltrials.gov IDs NCT01626495 agus NCT01029366) inar déileáladh le 25 leanbh (aois 5-22) agus 5 duine fásta (aois 26-60) le R/R BCP-ALL agus T-cell ALL. Ach tá na torthaí tuar dóchais inti (freagra iomlán [CR] i 90%, loghadh marthanach le maireachtáil saor ó imeacht 6 mhí i 67%, agus ráta marthanais foriomlán [OS] de 78% [leanúint airmheánach, 7 mí; raon, 1-24 mí]).
Chuir staidéar TOWER (Céim 3, Randomized, Staidéar Lipéad Oscailte a Imscrúdaíonn Éifeachtúlacht an Antashubstaint BITE Blinatumomab Versus Caighdeán Cúraim Cheimiteiripe in Ábhair Aosach le Athiompaithe / Teasfhulangacha B-Réamhtheachtaithe GACH; clinictrials.gov aitheantóir NCT02013167) i gcomparáid le héifeachtaí trom-blinatumomab monaiteiripe RCP-R. Toisc go raibh daoine ag maireachtáil níos faide, cuireadh stop leis an staidéar go luath. Bhí AEanna sa ghrúpa blinatumomab mar an gcéanna leo siúd a chonacthas i staidéir níos luaithe, agus bhí rátaí AE coigeartaithe nochta níos ísle ag blinatumomab ná SOC.34 Oibríonn Blinatumomab freisin do dhaoine le Ph-dearfach, R/R BCP-ALL agus do leanaí le Ph-diúltach, R/R BCP-ALL.
30% to 50% of people with BCP-ALL in complete hematologic remission show persistent MRD. In the single-arm, phase 2 BLAST study (A Confirmatory Multicenter, Single-Arm Study to Assess the Efficacy, Safety, and Tolerability of the BiTE Antibody Blinatumomab in Adult Patients With MRD of B-Precursor Acute Lymphoblastic Leukaemia; clinicaltrials.gov identifier NCT01207388), blinatumomab was tested on patients with BCP-ALL in first or later complete After blinatumomab treatment, 78% of patients who were MRD positive became MRD negative. The 5-year OS study showed a median OS of 36.5 months, and more than half of those who had a complete MRD response after the first cycle of blinatumomab were still alive at 5 years, which suggests that the treatment might be able to cure some patients. AEs were seen that were linked to siondróm scaoileadh cytokine (CRS).31 Other studies, like NCT03023878 and NCT03340766, are still looking at blinatumomab in first-line settings and in combination with other treatments.
CD19-targeted treatments have been linked to failure because of the loss of CD19 antigen after treatment. The failure rates for blinatumomab range from 8% to 35%, and for Teiripí T-chealla CAR, they range from 39% to 65%.36-40 We don’t fully understand what causes therapy to fail, but one possibility is immunoediting, in which antigen loss is caused by a T-cell-dependent process called immunoselection, which lets tumour cells get away.41 Lineage switch and epitope loss under therapy pressure have also been suggested as ways for tumours to escape treatment. However, a recent study on epitope loss found that some CD19 isoforms that help CAR T-cells escape were already present at the time of diagnosis. This suggests that combining treatments might be helpful. Another thing that can cause immunotherapy to fail is called “inhibitory T-cell signalling.” In this case, the blocking programmed death ligand-1 (PD-L1) is interesting because it is more common in B-cell ALL cells from patients who don’t respond to blinatumomab and can make CD3 BiTE molecules less effective.43 By making a CD28/PD-L1 BiTE that triggers the CD28 co-stimulatory signal instead of the inhibitory signaling pathway that is usually seen when a T cell binds to a PD-L1-expressing cancer cell, this inhibition could be turned off.43 Dual-targeted CAR T cells are also being looked into as a way to make up for the loss of tumour antigens. This can be done by modifying each T cell with 2 CAR molecules and 2 different binding domains (dual-signaling CAR) or by putting 2 different binding domains on 1 CAR molecule at the same time (TanCAR).
Imeachtaí díobhálacha le BiTE agus a bhainistíocht
I staidéir chliniciúla ar blinatumomab, is iad na AEanna is coitianta ná fiabhras, líon íseal cille fola bán, agus líon íseal pláitíní. Ar chuid de na rioscaí is tábhachtaí tá CRS, néarthocsaineacht, agus botúin drugaí. Is féidir le néarthocsaineacht tarlú freisin le cóireálacha CAR T-chealla a bhaineann go sonrach le CD19, ach b’fhéidir nach mar gheall ar CD19 atá sé. Léirigh torthaí staidéar chéim 1/1b atá fós ar siúl faoi spriocanna CD20/CD3 go raibh AEanna CNS de ghrád 3 nó níos airde annamh (3% de gach AE grád 3). An chuid is mó den am, bíonn imoibriú blinatumomab ar CRS éadrom, ach i gcásanna neamhchoitianta, is féidir leis a bheith dian agus fiú bagrach don bheatha. Is féidir frithghníomhartha athlastacha a laghdú le corticosteroidí. Chun an seans CRS a ísliú, is fearr insileadh de prednisone nó dexamethasone a thabhairt roimh an gcéad dáileog de blinatumomab agus an dáileog a mhéadú go mall. Thug an úsáid seo de corticosteroids roimh mhóilíní BiTE eile cúis le húsáid dexamethasone mar réamhchógais nuair a úsáidtear móilíní BiTE eile. Mar sin féin, níl sé soiléir an féidir an éifeacht seo a chur i bhfeidhm ar an ardán iomlán BiTE, agus táthar ag breathnú ar bhealaí eile chun déileáil le CRS. Is cítocín é Interleukin 6 a chruthaíonn CRS agus tá sé ard i ndaoine a bhfuil sé. Baineadh úsáid as Tocilizumab, a bhlocálann an gabhdóir interleukin-6, chun cóireáil a dhéanamh ar CRS atá an-dona tar éis cóireáil T-chealla CAR.49 San ospidéal, baineadh úsáid as coscairí neacróis meall chun CRS a chóireáil.


