Car T-Cell Therapy In Turkey
Kuronga kushanyira Turkey kune CAR T kurapwa?
Wana fungidziro kubva kuzvipatara zvepamusoro muTurkey.
CAR T cell therapy iri kubuda munzvimbo yeTurkey yehutano, ichipa tariro itsva kune varwere vane mamwe magomarara eropa. Kurapa kwechizvino-zvino uku kunosanganisira kushandura masero ezvirwere zvemurwere kuti anange maseru egomarara nemazvo. Ndichiri kusimukira, nzvimbo dzekurapa dzeTurkey dziri kuongorora kugona uye kushanda kweCAR T cell therapy. Zvinetso zvakaita semutengo uye zvivakwa zviripo, asi kutsvagisa kuri kuramba kuchienderera mberi nekudyidzana kunoratidza kufarira kuri kukura kwekutora iyi vimbiso yekurapa kusimudzira kutarisirwa kwegomarara muTurkey.
NOKUTI-T kurapa inzira itsva yekurapa cancer iyo inoshandisa immune system kupedza maseru egomarara. Uko kumwe kurapa kwakakundikana, kwave kuchikwanisa kuporesa apo neapo. Iyi blog inosimbisa zvese zvaunoda kuziva nezve iyi maitiro. Verenga kuti uwane zvimwe!
Chii chinonzi CAR-T Cell therapy?
Rudzi urwu rwekurapa runosanganisira kushandura masero eT emurwere, rudzi rwesero rekudzivirira, murabhoritari kuitira kuti vasunge nekuuraya maseru egomarara. Chubhu inotakura ropa kubva mutsinga iri muruoko rwomurwere kuenda kune imwe apheresis (isina kuratidzwa), iyo inobvisa masero machena eropa, kusanganisira T masero, uye inodzorera ropa rasara kumurwere.
Masero eT anobva agadziridzwa murabhoritari kuti ave nejene reiyo yakasarudzika inozivikanwa sechimeric antigen receptor (CAR). Masero eCAR T anowanzwa murabhoritari asati apinzwa mumurwere akawanda. Iyo antigen pamasero ecancer inogona kuzivikanwa neCAR T masero, ayo anobva auraya maseru egomarara.
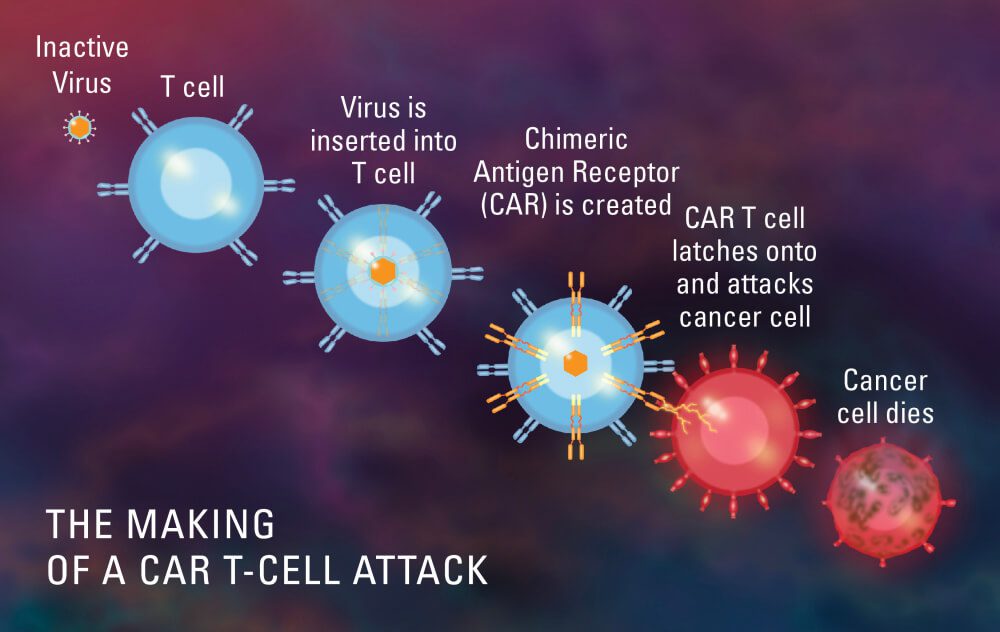
Ndeipi nzira yeCAR-T Cell therapy?
Iyo CAR-T kurapwa maitiro, iyo inotora mavhiki mashoma, inosanganisira akawanda matanho:
T masero anotorwa muropa rako uchishandisa chubhu inoiswa mutsinga yeruoko. Izvi zvinotora maawa mashoma.
Masero eT anotakurwa kuenda kunzvimbo uko anogadziridzwa genetic modified kuti ave masero eCAR-T. Vhiki mbiri kana nhatu dzinopfuura mukati meizvi.
Masero eCAR-T anopinzwazve muropa rako kuburikidza nedrip. Izvi zvinoda maawa akawanda.
Masero eCAR-T anotarisa uye anobvisa masero egomarara mumuviri wese. Mushure mekugamuchira CAR-T kurapwa, iwe unozotariswa zvakanyanya.
Ndezvipi zvinokonzerwa neCAR-T Cell therapy?
Cytokine kuburitsa chirwere, or CRS, is the typical CAR T-cell side effect. Another name for it is “cytokine storm.” It is experienced by roughly 70–90% of patients, but it only lasts for five to seven days. The majority of people compare it to having a bad flu infection, complete with a high fever, exhaustion, and bodily aches.
Zuva rechipiri kana rechitatu rinotevera infusion kazhinji parinotanga. Izvo zvinoitika nekuda kwesimba remuviri rekudzivirira rinoita kune T masero 'kuwanda uye kurwisa hutsinye.
CRES, inomirira CAR T-cell-related encephalopathy syndrome, ndiyo imwe mhedzisiro yakaipa. Munenge zuva rechishanu rinotevera infusion, inowanzotanga. Varwere vangave vachivhiringika uye kuvhiringidzika, uye pano neapo vangatadza kutaura kwemazuva akawanda.
Kunyangwe CRES ichidzokororwa uye kazhinji inotora mazuva maviri kusvika mana, inogona kunetsa kuvarwere nemhuri dzavo. Yese mabasa etsinga zvishoma nezvishoma anodzokera kune akajairika muvarwere.
Imhando ipi yemaseru egomarara anogona kurapwa neCAR-T Cell Therapy?
Only patients with adult B-cell non-lymphoma Hodgkin’s or pediatric acute lymphoblastic leukemia who have already tried two unsuccessful conventional therapies can currently use CAR T-cell therapy products that have received FDA approval. However, CAR T-cell therapy is now being tested in clinical studies as a first or second-line treatment for adult lymphoma and pediatric acute lymphoblastic leukemia.
Ndezvipi zvakanakira zveCAR-T Cell therapy?
Chakanyanya kubatsira ndechekuti CAR T-cell therapy inongoda kuisirwa kamwe chete uye kazhinji inongoda mavhiki maviri ekuchengetwa kwevarwere. Varwere vane isiri-Hodgkin lymphoma uye leukemia yevana vachangobva kuongororwa, kune rumwe rutivi, vanowanzoda chemotherapy kweinenge mwedzi mitanhatu kana kupfuura.
Zvakanakira zveCAR T-cell therapy, inova iri mushonga mhenyu, inogona kuramba iripo kwemakore mazhinji. Kana uye kana kudzokazve kukaitika, maseru anozokwanisa kuona uye kunanga maseru egomarara nekuti anogona kurarama mumuviri kwenguva yakareba.
Although the information is still developing, 42% of adult lymphoma patients who underwent CD19 CAR T-cell treatment were still in remission after 15 months. And after six months, two-thirds of patients with pediatric acute lymphoblastic leukemia were still in remission. Unfortunately, these patients had exceedingly aggressive tumors that weren’t successfully treated using traditional standards of care.
Ndeupi rudzi rwevarwere vangave vagamuchire vakanaka veCAR-T Cell Therapy?
Munhu anonyatsokwanisa kumirira CAR T-cell therapy panguva ino mwana ane acute lymphoblastic leukemia kana munhu mukuru ane B-cell lymphoma yakaoma uyo anga atova nemitsara miviri yekurapa kusingashandi.
Gore ra2017 risati rapera, pakanga pasina mwero wakagamuchirwa wekutarisira varwere vanga vatopfuura nemitsetse miviri yekurapa vasina kuwana kuregererwa. Mushonga chete wakatenderwa neFDA kusvika parizvino waratidza kubatsira zvakanyanya kuvarwere ava ndeyeCAR T-cell therapy.
Ndeipi chiyero cheCAR-T Cell therapy muTurkey?
A pilot kliniki yekuedzwa (NCT04206943) designed to assess the safety and feasibility of ISIKOK-19 T-cell therapy in patients with relapsed and refractory CD19+ tumors was conducted and participating patients received ISIKOK-19 infusions between October 2019 and July 2021. Production data of the first 8 patients and the clinical outcome of 7 patients who received ISIKOK-19 cell infusion is presented in this study.
Results: Vapfumbamwe varwere vakanyoreswa kuedzwa (ALL n = 5 uye NHL n = 4) asi varwere ve7 chete vaigona kugamuchira kurapwa. Vaviri kubva pavatatu VOSE varwere uye vatatu kubva kune vana varwere veNHL vaiva nemhinduro yakakwana / chikamu (ORR 72%). Varwere vana (57%) vaive neCAR-T-yakabatana toxicities (CRS, CRES, uye pancytopenia). Varwere vaviri vaive vasingadaire uye vaive nechirwere chinoenderera mberi zvichitevera kurapwa kweCAR-T. Varwere vaviri vane chikamu chemhinduro vaive nechirwere chinopfuurira panguva
tevera.
mhedziso: Kubudirira kwekugadzira uye kuzadzisa maitiro ekutonga kwemhando kwaigutsa pakugadzirwa kwedzidzo. Response rates uye toxicity profiles zvinogamuchirwa kune iri rakanyanya pretreated/refractory murwere boka. ISIKOK-19 maseru anoita seyakachengeteka, ine mari, uye inoshanda sarudzo yekurapa yemamota eCD19 positive. Zviwanikwa zvechidzidzo ichi zvinofanirwa kuve
inotsigirwa nechirongwa chekiriniki chirikuenderera mberi cheISIKOK-19.
Kupedza
Izvi zvinomiririra kufambira mberi kwakakosha mukutarisira kweleukemia uye B-cell lymphoma. Uyezve, inopa tariro kune avo vaimbofanotaurwa kuti vaizogara mwedzi mitanhatu chete. Zvino zvataona nzira dzekuramba uye nekugadzira mamwe matekiniki ekuzvirwisa, ramangwana rinoita serinonyanya kuvimbisa.
Kuti uwane rumwe ruzivo nezve CAR-T Cell Therapy muTurkey, tungamira kune yedu Website. Bata nevatapi vedu vane ruzivo rwakanyanya pano paCancerFax kuti uwane kubvunzurudzwa kwemahara kuti ugadzire chirongwa chakakodzera chekuchengetedza zvido zvako zvehutano!

Mufananidzo: Chimwe chechipatara muTurkey uko CAR T Cell therapy miedzo yakaitwa.