
CAR T-Cell Therapy in India
In October 2023, the Central Drugs Standard Control Organization (CDSCO), which is India’s equivalent of the US Food and Drug Administration, granted approval to NexCAR19, making it the first CAR-T cell therapy to be licensed in India. CAR T Cell therapy in India has been officially launched in 6 hospitals across Delhi, Mumbai, and Pune.
In the past few years, Indian hospitals and study centres have come a long way towards using CAR T-cell therapy. CAR T-cell therapy could change the way cancer is treated, so it gives patients who don’t have many other choices new hope. In this new treatment, the patient’s own immune cells are reprogrammed to find and kill cancer cells.
CAR T-Cell Therapy MuIndia – Current Status
February, 2024: In October 2023, the Central Drugs Standard Control Organization (CDSCO), which is India’s equivalent of the US Food and Drug Administration, granted approval to NexCAR19, making it the first CAR-T cell therapy to be licensed in India. CAR T Cell kurapa muIndia has been officially launched in 6 hospitals across Delhi, Mumbai, and Pune.
Rezinesi rakapihwa zvichibva pane zvakawanikwa kubva kuongororo mbiri dzekiriniki dzakaganhurirwa dzakaitwa muIndia dzinosanganisira vanhu makumi matanhatu nevana vakawanikwa vaine advanced lymphoma kana leukemia. Zvichienderana nezvakawanikwa muyedzo wakapihwa kumusangano weAmerican Society of Hematology muna Zvita 64, zvakaonekwa kuti 2023% yevarwere (67 kubva pa36) vakapinda muzvidzidzo zviviri izvi vakawana kudzikira kwakanyanya muhukuru hwegomarara ravo (chinangwa chemhinduro. ) Inenge hafu yevarwere ava vakawana kunyangarika zvachose kwehuipi (mhinduro yakakwana).
ImmunoACT, inotsigira IIT Bombay, yakapa mari yekuyedza uye ichave nebasa rekugadzira uye kushambadzira kwe actalycabtagene autoleucel.
Apart from current ongoing clinical trials in ACTREC and Narayana, Bengaluru, MGRC has collaborated with a China-based CAR cell biotech company to bring CAR T-Cell therapy to India. At present, this life-saving therapy is available in the USA, UK, Canada, Israel, Singapore, China, Malaysia, & Australia. The cost of this therapy is around 5-7,00,000 USD in the USA, whereas in China it costs anywhere between $70,000 uye $80,000 USD.

Miedzo yemakiriniki ye MOTO T- Cell kurapwa for the treatment of some types of gomarara reropa have kicked off at the Advanced Centre for Treatment, Research, and Education in Cancer, the research and development wing of Tata Chirangaridzo Center. “More details of the trial will be revealed soon,” Dr. Narula said in a press brief. This kliniki yekuedzwa is taking place with the help of a researcher from IIT, Bombay, who has developed this life saving therapy.
Dr. Reddys lab has also secured a deal with Shenzen Biopharma Pregene of China on May 21 to bring this life-saving therapy to India. There are several other companies that are also working to bring this technology to India. US-based Indian-born oncologist Dr. Siddharth Mukherjee was in India recently and had a meeting with Kiran Mazumdar Shaw of Biocon & Mr. Kush Parmar of 5 AM Ventures. All of them have agreed to come up with a facility to grow a Chimeric Antigen Receptor (CAR) cells to fight cancer. As per the reports, this therapy can be available in India in about a year’s time. This therapy has recently been approved by FDA (Food and Drug Administration). This cell therapy is useful for treatment in certain children and young adults suffering from Non – Hodgkin lymphoma. Treatment with Yeskarta & Kymriah is the first CAR T-Cell therapy to receive FDA approval.
Kunyangwe paine chokwadi chekuti kune akawanda masero ekurapa emakiriniki miedzo inoshanda uye inonyoresa muUnited States, European Union, neChina, hapana imwe yakawanikwa muIndia.
The itsva Immuneel facility in Bengaluru’s Narayana Health City is dedicated to introducing high-quality and affordable cell therapies to India. The facility’s strategic location in a tertiary care hospital near a high-volume bone marrow transplant unit allows for further coordination between research teams and clinicians, which is important for focused clinical development of innovative personalized therapies like CAR-T.

Immuneel is working hard to advance its pipeline. The company’s strategy of licensing a CAR-T asset that has already been clinically tested is expected to result in the company’s first cell therapy clinical trial in 2021. In terms of laboratory and production facilities, including equipment and instruments, Immuneel’s integrated facility is among the best in the world. This helps physicians and scientists work together seamlessly both internally and with research institutes across the world on product creation and distribution. To support this target, the organization has attracted exceptional global talent with prior experience in cell therapy, as well as a distinguished Scientific Advisory Board consisting of the field’s most respected scientific and intellectual giants.
These therapies are labor-intensive, meticulously managed, require costly reagents and consumables, and are difficult to automate. The logistics of preserving and transporting cryopreserved cells continues to be a global problem. Because of all of these factors, cell therapies are exceedingly difficult to produce and supply and, thus, extremely costly. Cell therapies are difficult to prescribe clinically, and patients must be closely monitored for adverse events in the hospital immediately after infusion.
Mhedzisiro Yechikamu II CAR T-Cell Therapy Clinical Trials MuIndia
PaASCO, Zvita 22 musangano, Immuneel, sero uye gene therapy kutanga, yakazivisa kuti zvekutanga zvakawanikwa kubva muIndia yekutanga chikamu 2 kiriniki miedzo yakaratidza 77% yehuwandu hwekupindura kwemazuva makumi mapfumbamwe muvarwere vane kenza yeropa senge leukemia nelymphoma. Immuneel iri kugadzira iyo CAR-T cell therapy Varnimcabtagene.
Zviwanikwa zvekutanga kubva muyedzo yeIMAGINE zvakabva pakunyoreswa kwevanhu vekutanga gumi kubva muhuwandu hwevarwere ve10.
Pazuva 28, pamusoro pe80% yevarwere vaive nekupora kuzere kwekiriniki. PaZuva 90, iyo IMAGINE data yakaratidza huwandu hwekupindura huwandu hwe77%, nemhinduro dzakakwana dzichiratidzwa mu6 kubva ku9 varwere vanoongororwa.
The B acute lymphoblastic leukemia varwere 'day 28 and day 90 readouts inoratidza 100% uye 83% yekuregererwa kwakakwana, zvichiteerana, zvichiratidza mhinduro dzinokurumidza, dzakasimba, uye dzinogara kwenguva refu.
Varnimcabtagene yakatora mazuva gumi nemaviri paavhareji kugadzira uye kuburitsa, ine 12% yekugadzira budiriro.
Kiran Mazumdar-Shaw, sachigaro weBiocon, Siddhartha Mukherjee, ane mukurumbira oncologist uye munyori, uye Kush Parmar, maneja mudiwa we5AM Venture, akabatana neImuneel. Imuneel iri kugadzira pombi yayo ye chimeric antigen receptor T-cell (CAR-T) marapiro uye mamwe ma cellular immunotherapies ekurapa cancer.
Chii Chiyero CheCar-T Cell Therapy muIndia?
Introduction: CAR T- cell therapy rudzi rutsva rwekurapa rwuri kushandura marapirwo anoitwa gomarara pasi rose. Mumakore mashoma apfuura, India yakafambira mberi zvakanyanya mukutora kurapwa uku, ichipa varwere vane mhando dzakasiyana dzegomarara zvikonzero zvitsva zvekutarisira. CAR-T cell therapy inogona kuve nemhedzisiro yakakura pahutano hweIndia nekuti inogona kushandura mabatirwo egomarara.
Kuwedzera Kurapa Sarudzo: Kuuya kweCAR-T cell therapy kuIndia kwakapa varwere nzira dzakawanda dzekurapwa, kunyanya avo vane gomarara reropa senge leukemia nelymphoma. Kurapa uku kunosanganisira kutora T masero emurwere, achiachinja genetically kuti abudise chimeric antigen receptors (CARs) akananga maseru egomarara, obva aadzosera mumuviri wemurwere. CAR-T cell therapy inzira yakasarudzika inoita kuti zvive nyore kuti immune system iwane nekuuraya maseru egomarara.
Kutsvakurudza nekuvandudza: R&D has come a long way. India has a strong infrastructure for research and development, and top institutions and hospitals are actively looking into the potential of CAR-T cell therapy. This dedication to study has led to exciting new developments, such as the creation of CAR-T cell therapies that are tailored to the unique genetic and ethnic diversity of the Indian people. These kinds of improvements help to broaden the therapy’s reach and make it possible to use it on more types of cancer.
Kukwanisa uye kuwanikwa: Chimwe chezvinhu zvakanakisa nezve CAR-T sero kurapwa muIndia ndechekuti, zvichienzaniswa nedzimwe nyika dzekuMadokero, inokwanisika. Varwere vanobva kumativi ese ehupenyu vanogona kunge vachikwanisa kuzvitenga nekuti mitengo yakaderera uye kune dzakawanda sarudzo dzekurapa. Zvakare, huwandu hwezvipatara zveIndia uye nzvimbo dzekurapa dzakashanda nemakambani epasi rese ezvinodhaka kuti vauye neCAR-T cell kurapwa kuIndia, zvichiita kuti zvive nyore kune vanhu vanoda kuiwana.
Matambudziko uye Tarisiro Yeramangwana: CAR-T cell treatment ine zvakawanda zvingaita, asi inewo mamwe matambudziko. Zvimwe zvezvinetso zvinoda kugadziriswa kudhura kwekurapa, kuoma kwekugadzira mushonga, uye kudiwa kwemichina inodiwa. Asi hurumende yeIndia, ichishanda nevane chekuita nezvehutano, iri kushanda nesimba kugadzirisa matambudziko aya uye nekugadzira hurongwa hwekuti kurapwa kushandiswe nenzira yakachengeteka uye inoshanda.
Kushandiswa kweCAR-T cell therapy muIndia kuri kukura nokukurumidza, kupa varwere vekenza tariro itsva uye nzira dziri nani dzekurapa chirwere chavo. India inzvimbo yakanaka yekurapa kwepasi nekuti yakazvipira kudzidza, mitengo yakadzikira, uye kuwana kuri kuita nani. Sezvo hurongwa hwekuchengetedza hutano hweIndia huri kuramba huchichinja, kuwedzerwa kweCAR-T cell therapy kungangoshandura mabatirwo egomarara, zvichiita kuti zvinhu zvive nani kuvarwere uye kuchinja hupenyu.
Where is CAR T Cell therapy available in India?
You can find CAR T-Cell therapy in several prominent Indian medical healthcare centers, such as Tata Memorial Centre, Apollo Cancer Hospital, BLK, Artemis, Asian Oncology, American Oncology, and HCG.
Kuvepo kweCAR T Cell Therapy munzvimbo idzi dzine mukurumbira dzehutano inhanho huru mukurwisa gomarara kweIndia. Tsvaga zvipatara zve5 zvinozivikanwa nekupa zvakanakisisa CAR-T treatment in India.
Tata Memorial Cancer Hospital muMumbai
Pakati pevanotungamira vanopa ve CAR T Kurapa MuIndia, first comes Tata Memorial Hospital, which is a world-class cancer treatment provider. In this hospital, a team of experienced doctors and researchers work hard to fight cancer using advanced treatments. The hospital is known for its excellence in cancer care and has a lot of experience in using CAR T Cell therapy to help patients get better. People come here from all over because they trust Tata Memorial to give them the best chance at beating cancer. So, if you or someone you know needs excellent cancer care, Tata Memorial Cancer Hospital is a great choice.
Apollo Cancer Institute muChennai
Apollo Cancer Institute muChennai inzvimbo inozivikanwa yehutano inozivikanwa neakanakisa masevhisi ekurapa cancer. Vanopa hutano hwakakwana kuvarwere vegomarara kuburikidza nehunyanzvi hwepamusoro uye timu ine ruzivo rwakanyanya yeoncologists. Chikwata chavo chakazvipira chevanooncologists, vanachiremba vekuvhiya, uye vashandi vekutsigira vanoshanda nesimba kugadzira zvirongwa zvekurapa zvemunhu zvinoisa pamberi pese kushanda uye hupenyu hwemurwere. Kuzvipira kwavo kuita zvakanaka kwakaita kuti vave nemukurumbira unoremekedzwa mumunda wekuchengeta cancer.
National Cancer Institute (AIIMS) muDelhi
Iyo National Cancer Institute (AIIMS) muDelhi ndeimwe inotungamira nzvimbo yeCAR-T cell therapies. Unogona kuwana mukana weCAR-T kurapwa pano nemutengo unodhura kwazvo. Ichi chikoro chekurapa chinozivikanwa nekutsvagisa kwayo kwepamusoro pa immunoadoptive cell therapy, nzvimbo dzepamusoro-notch, uye vanachiremba vane hunyanzvi. Nzvimbo iyi inotora nzira yechikwata, ichiunza pamwe chete vane ruzivo oncologists, maoparesheni, radiologists, uye vashandi vekutsigira kugadzira zvirongwa zvekurapa zvegomarara reropa pamwe nemamwe marudzi ese egomarara. Ivo vanotoshandisa tekinoroji yepamberi senge hungwaru hwekugadzira uye genetic ongororo kuti vape yakanakisa cancer kuchengetedza kuvarwere.
BLK Max Cancer Center, Delhi
The BLK Max Cancer Centre in Delhi is one of India’s leading cancer hospitals, dedicated to providing chimeric antigen receptor t cell therapy (CAR T). Their institute has advanced technology for cancer care, which includes robotic surgery, tomo therapy, and immunotherapy. Their warm and supportive environment can help you stay strong and combat the disease.
Rajiv Gandhi Cancer Institute & Research Center muDelhi
Rajiv Gandhi Cancer Institute & Research Center muDelhi ndiyo imwe yenzvimbo dzinotungamira kenza muAsia. Tekinoroji yavo yehunyanzvi uye vashandi vane tarenda vanopa hutano hwepasi rose hwegomarara kuvarwere muIndia, pamwe nemunyika dzeSAARC. Sangano iri rakava nerukudzo rwekukurudzira hupenyu hwevarwere vegomarara vangangoita 2.75 lakh kubva payakavambwa muna 1996. Nyanzvi yavo inopa rubatsiro rwakanyanya kuvarwere vegomarara kuburikidza neCAR T Cell therapy inodhura muIndia.
Sangana Naivo Vepamusoro Vanorapa VepaCAR T Cell Therapy MuIndia
Dzidza nezve epamusoro oncologists ye CAR T Kurapa MuIndia. Vanachiremba vane ruzivo ava vakazvipira kupa yakanakisa sero kurapwa kwegomarara pamwe nekutarisirwa kwemunhu nerutsigiro. Vimba nehunyanzvi hwavo kune ramangwana rakajeka mukurwira kwako gomarara!
Dr T Raja (MD, DM)
Dr. T Raja ndiye anozivikanwa Medical Oncologist ane makore anopfuura 25 ane ruzivo mukurapa kenza. Iye anozivikanwa neruzivo rwake rwakatanhamara uye anoonekwa semumwe weIndia yepamusoro oncologists. Dr. Raja anewo chinzvimbo chepamusoro chedzidzo, achitungamira chirongwa cheDNB Medical Oncology paApollo Specialty Hospital muChennai. Iye mutauri anotsvakwa pamisangano yenyika uye yepasirese, kwaanogovera ruzivo rwake rwakakosha.
Dr Srikanth M (MD, DM)
Dr. Srikanth M. inyanzvi yehematologist ane ruzivo rwakanyanya muChennai, anozivikanwa nehunyanzvi hwake mukurapa zvirwere zvakasiyana-siyana zvine chekuita neropa. Anoshanda mune zvese zvenguva pfupi uye zvenguva refu nyaya dzehutano seanemia, myeloma, b-cell lymphomas uye leukemia. Dr. Srikanth M. anopawo zviedzo zvepamusoro kuti uongorore hutano hwako hwose, zvakadai semapfupa emapfupa uye chelation therapy yezviitiko zvisingawanzoitiki zvakadai sekuwedzera kwemaminerari muropa. Dr. Srikanth M. akagamuchira mibairo yezvipo zvake pakutsvakurudza kwemyeloma, zvichiita kuti ave nyanzvi inovimbwa muhematology yakagadzirira kupa rubatsiro rwakanyanya kuvarwere vanoda rubatsiro.
Dr Revathi Raj (MD, DCH)
Dr. Revathi Raj inyanzvi inoremekedzwa zvikuru inozivikanwa nehunyanzvi hwayo mukurapa mapfupa evana. Akabudirira kuita zvinopfuura 2000 zveaya ma transplants, achimuita nyanzvi inotungamira yeIndia. Dr. Raj ane ruzivo rwakakura rwokurapa vana vane zvirwere zveropa zvakadai sethalassemia, hemophilia, sickle cell anemia, aplastic anemia, uye leukemia. Akazvipira zvakanyanya kukugara zvakanaka kwevana, achimhanyisa sevhisi yekurapa leukemia yevana uye lymphoma ine 80% yekurapa.
Cost Of Car T-Cell Treatment In India
Musi waGumiguru 13, 2023, kambani inonzi Immunoadoptive Cell Therapy Private Limited (ImmunoACT) muMumbai yakagamuchira mvumo kubva kuCentral Drugs Standard Control Organisation (CDSCO) yeIndia yekutanga kurapwa kenza inonzi NexCAR19.
Mushonga uyu wakanyatsogadzirirwa kubatsira vanhu vane mamwe marudzi eukemia uye lymphoma vasina kupindura kune mamwe marapirwo. Chiyero chekiriniki chechiitiko ichi chakaitwa pavarwere makumi matanhatu vane lymphomas uye leukamia. Huwandu hwekupindura kweiyi yakakosha kuyedzwa kwekiriniki yaive 60% iyo yakairatidza senge yakabudirira kurapa kuparadza maseru egomarara zvakanyanya.
The mutengo weCAR T cell kurapwa muIndia is approximately USD 57,000. This price is much lower when compared to countries like the USA, Singapore, Malaysia etc. However, it’s crucial to note that this pricing can vary depending on a variety of factors. The CAR-T cell therapy costs might differ from one hospital to another depending on their technology, expertise, and other facilities.
Furthermore, the type of CAR T-cell therapy required and the condition of the patient may also affect the overall cost.
Chii Chinonzi Mota T-Cell Therapy?
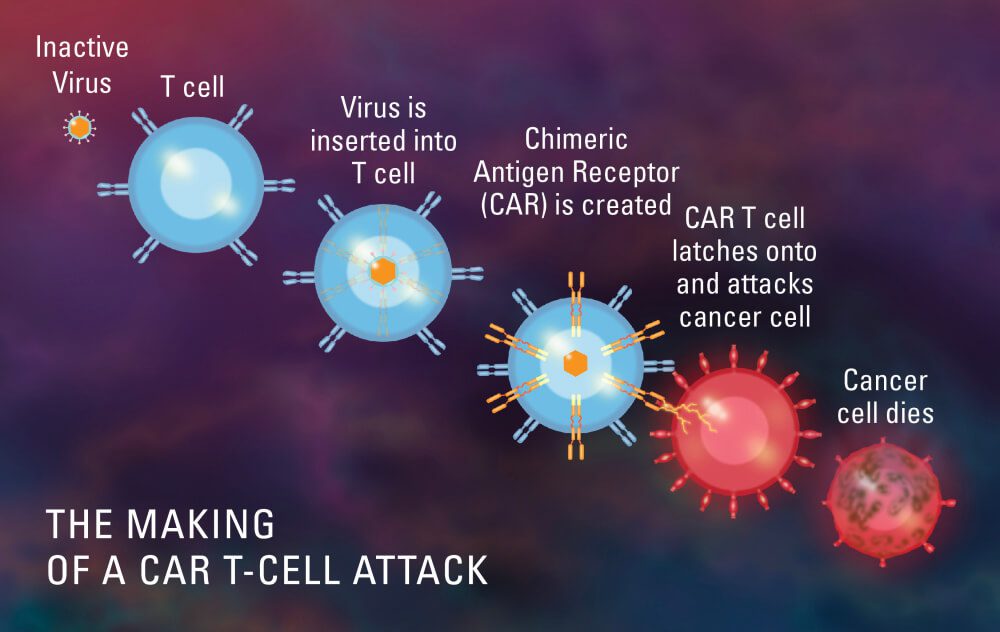
Chimeric antigen receptor T-cell therapy, inowanzozivikanwa seCAR T-cell therapy, ivhu-inoputsa immunotherapy iyo yakashandura zvachose nzira iyo kenza inorapwa. Inopa varwere vane mamwe magomarara tariro yaimboonekwa seisingarapike kana kuti ine nzira shoma dzekurapa.
Kurapa uku kunosanganisira kushandisa masero emuviri emuviri wemurwere-kunyanya, T masero-uye kuachinja-chinja kuvandudza kugona kwavo kuona nekuparadza maseru egomarara. Kuti aite izvi, masero eT anopihwa chimeric antigen receptor (CAR), iyo inovapa kugona kunanga mamwe mapuroteni, kana maantigen, pamusoro pemasero egomarara.
Masero eT kubva kumurwere anotanga kubviswa, uye anobva agadziriswa genetically kuratidza CAR. Murabhoritari, masero akachinjwa aya anowanzwa kuti abudise huwandu hwakati wandei hwemasero eCAR T, anozodzoserwa muropa remurwere.
Pavanenge vari mukati memuviri, masero eCAR T anowana masero ekenza anoratidza antigen inodiwa, anonamatira paari, uye anokonzera simba rekudzivirira zvirwere. Masero eCAR T akabatiswa anowedzera uye anoita kurwisa kwakanangana nemasero egomarara, achiauraya.
Kana ichishandiswa kurapa mamwe maronda eropa senge acute lymphoblastic leukemia (ALL) uye chaiwo mafomu e lymphoma, CAR T-cell therapy yakaratidza mhedzisiro. Yakaburitsa mhinduro dzinozivikanwa uye mune vamwe varwere, kunyangwe kuregererwa kwenguva refu.
CAR T-cell therapy, zvisinei, inzira yakaoma uye yakasarudzika yekurapa inogona kuve nenjodzi nemhedzisiro yakaipa. Cytokine release syndrome (CRS), yakapararira immunological reaction iyo inogona kukonzera zviratidzo-sefuruwenza uye, mumamiriro ezvinhu akaipisisa, kutadza kwenhengo, kunogona kuitika kune vamwe vanhu. Pakave zvakare nemishumo yezvinokonzeresa neurological yakashata, zvisinei iyo inowanzorapika.
Zvisinei nematambudziko aya, CAR T-cell therapy ibudiriro huru mukurwisa gomarara uye inoratidza kugona kukuru kweramangwana. Zvidzidzo zvazvino zvinonangana nekusimudzira kushanda kwayo uye kuchengetedza chimiro pamwe nekuwedzera mashandisiro ayo kune akasiyana marudzi egomarara. CAR T-cell therapy ine kugona kushandura chiso chekurapa cancer uye kupa varwere kwese kwese tariro itsva nekumwe kufambira mberi.
nzira
Iyo CAR-T kurapwa maitiro, iyo inotora mavhiki mashoma, inosanganisira akawanda matanho:
T masero anotorwa muropa rako uchishandisa chubhu inoiswa mutsinga yeruoko. Izvi zvinotora maawa mashoma.
Masero eT anotakurwa kuenda kunzvimbo uko anogadziridzwa genetic modified kuti ave masero eCAR-T. Vhiki mbiri kana nhatu dzinopfuura mukati meizvi.
Masero eCAR-T anopinzwazve muropa rako kuburikidza nedrip. Izvi zvinoda maawa akawanda.
Masero eCAR-T anotarisa uye anobvisa masero egomarara mumuviri wese. Mushure mekugamuchira CAR-T kurapwa, iwe unozotariswa zvakanyanya.
Ndeupi Rudzi rweMasero eCancer Anogona Kurapwa neCar-T Cell Therapy?
Varwere chete vane B-cell vasiri lymphoma Hodgkin's kana pediatric acute lymphoblastic leukemia vakamboedza nzira mbiri dzekare dzisina kubudirira vanogona kushandisa zvigadzirwa zveCAR T-cell therapy izvo zvakagamuchirwa neFDA. Nekudaro, CAR T-cell therapy yave kuedzwa muzvidzidzo zvekiriniki seyekutanga kana yechipiri-mutsara kurapwa kwevakuru lymphoma uye yevana acute lymphoblastic leukemia. Nguva pfupi yadarika, zvimwe zvezvidzidzo zvakaratidza budiriro inoshamisa muzviitiko zvemamota akasimba senge glioblastoma, gliomas, kenza yechiropa, kenza yemapapu, kenza yeGI, kenza yepancreatic uye kenza yemumuromo.
Kupedza
Izvi zvinomiririra kufambira mberi kwakakosha mukutarisira kweleukemia uye B-cell lymphoma. Uyezve, inopa tariro kune avo vaimbofanotaurwa kuti vaizogara mwedzi mitanhatu chete. Zvino zvataona nzira dzekuramba uye nekugadzira mamwe matekiniki ekuzvirwisa, ramangwana rinoita serinonyanya kuvimbisa.
Bata nevatapi vedu vane ruzivo rwakanyanya pano pa CancerFax kubvunzurudzwa kwemahara kugadzira chirongwa chekuchengetedza chakakodzera chezvinodiwa zvehutano hwako. Ndokumbira utumire marepoti ako ekurapwa info@cancerfax.com kana WhatsApp kuti + 1 213 789 56 55.
Ndezvipi Zvakanakira zveCar-T Cell Therapy?
Chakanyanya kubatsira ndechekuti CAR T-cell therapy inongoda infusion imwechete uye kazhinji inongoda mavhiki maviri ekuchengetwa kwevarwere. Varwere vane non-Hodgkin lymphoma uye leukemia yevana vachangobva kuongororwa, kune rumwe rutivi, vanowanzoda chemotherapy kwemwedzi mitanhatu kana kupfuura.
Zvakanakira zveCAR T-cell therapy, inova iri mushonga mhenyu, inogona kuramba iripo kwemakore mazhinji. Kana uye kana kudzokazve kukaitika, maseru anozokwanisa kuona uye kunanga maseru egomarara nekuti anogona kurarama mumuviri kwenguva yakareba.
Kunyange zvazvo ruzivo ruchiri kusimukira, 42% yevarwere vakuru ve lymphoma vakawana CD19 CAR T-cell kurapwa vakanga vachiri mukuregererwa mushure memwedzi gumi nemishanu. Uye mushure memwedzi mitanhatu, zvikamu zviviri muzvitatu zvevarwere vane acute lymphoblastic leukemia vakanga vachiri mukuregererwa. Nehurombo, varwere ava vaive nemamota ane hasha asina kurapwa nenzira yechinyakare yekuchengeta.
Ndeupi rudzi rwevarwere vangave vagamuchire vakanaka veCAR-T Cell Therapy?
Varwere vari pakati peMakore matatu kusvika kuMakore makumi manomwe vakaedzwa neCAR T-Cell kurapwa kwemhando dzakasiyana dzegomarara reropa uye zvakaonekwa kuti zvinoshanda zvakanyanya. Nzvimbo zhinji dzakati budiriro inopfuura 3%. Munhu anonyatsokwanisa kumirira CAR T-cell therapy panguva ino mwana ane acute lymphoblastic leukemia kana munhu mukuru ane B-cell lymphoma yakakomba anga atova nemitsetse miviri yekurapa kusingashandi.
Gore ra2017 risati rapera, pakanga pasina mwero wakagamuchirwa wekutarisira varwere vanga vatopfuura nemitsetse miviri yekurapa vasina kuwana kuregererwa. Mushonga chete wakatenderwa neFDA kusvika parizvino waratidza kubatsira zvakanyanya kuvarwere ava ndeyeCAR T-cell therapy.
Car-T Cell Therapy Inoshanda Sei?
CAR T-cell therapy yave ichishanda zvikuru mukurapa mamwe marudzi egomarara reropa, seacute lymphoblastic leukemia (ALL) uye asiri Hodgkin lymphoma. Mumakiriniki ekuedzwa, mazinga ekupindura ave akanaka kwazvo, uye varwere vazhinji vakapinda mukuregererwa kwakakwana. Mune zvimwe zviitiko, vanhu vakanga vaedza mimwe mishonga yose vaiva nekuregererwa kwenguva refu kana kuti kurapa kunobvira.
Chimwe chezvinhu zvakanakisa nezveCAR T-sero kurapwa ndechekuti inonangana nemasero akakodzera. Iwo maCAR receptors akawedzerwa kumasero eT anogona kuwana chaiwo mamaki pamasero egomarara. Izvi zvinoita kuti zvikwanise kupa kurapa kwakanangwa. Iyi nzira yakanangwa inokuvadza maseru ane hutano zvishoma sezvinobvira uye inodzikisa njodzi yemhedzisiro inouya nemishonga yechinyakare senge chemotherapy.
Asi zvakakosha kuyeuka kuti CAR T-cell therapy ichiri nzvimbo itsva ichiri kuchinja. Vatsvagiri uye vanachiremba vari kushanda nesimba kuti vagadzirise matambudziko akaita semutengo wakakwira, mukana wemhedzisiro yakakomba, uye chokwadi chekuti inongoshanda kune mamwe marudzi egomarara.
Pakupedzisira, CAR T-cell therapy yakaratidza kuva nzira yakabudirira zvikuru yekurapa mamwe marudzi egomarara reropa. Kunyangwe iri nzira inovimbisa uye ine simba, kudzidza kwakawanda uye miedzo yekiriniki inodiwa kuivandudza nekutsvaga nzira nyowani dzekuishandisa. CAR T-cell therapy inogona kushandura marapirwo egomarara uye kuita kuti zvinhu zvirinani kuvanhu pasi rose kana ikaramba ichipora.
Kubatanidzwa & Kusabatanidzwa Criteria
Kusanganisa Maitiro eCAR T-Cell Therapy:
1. Varwere vane CD19+ B-cell Lymphoma (Anenge maviri asati asangana chemotherapy regimens)
2. Kuva ane makore matatu kusvika makumi manomwe nemashanu ekuberekwa
3. ECOG zvibodzwa ≤2
4. Vakadzi vane mukana wekubereka vanofanirwa kunge vane weti pamuviri bvunzo yakatorwa uye yakaratidza kusagadzikana pamberi pekurapwa. Vese varwere vanobvuma kushandisa nzira dzakavimbika dzekudzivirira kubata pamuviri panguva yekutongwa uye kusvika pakutevera kekupedzisira.
Maitiro ekusabatanidzwa eCAR T-Cell Therapy:
1. Intracranial neBP kana kufenda
2. Kutadza kufema
3. Yakaparadzirwa intravascular coagulation
4. Hematosepsis kana Utachiona husingadzoreki hunoshanda
5. Kusadzora chirwere cheshuga
Ndeapi Madivi Migumisiro YeCar-T Cell Therapy?
Pazasi pane mamwe emhedzisiro yeCAR T-Cell therapy.
- Cytokine release syndrome (CRS): Iyo yakanyanya kupararira uye ingangove yakakosha mhedzisiro yeCAR T-sero kurapwa ndeye cytokine release syndrome (CRS). Zviratidzo zvakaita sefuruu, zvinosanganisira fivha, kupera simba, kutemwa nemusoro, uye kurwadziwa kwemhasuru, zvinounzwa nekugadziridzwa kweT cell 'kugadzirwa kwemacytokines. Mumamiriro ezvinhu akanyanyisa, CRS inogona kukonzera kupisa kwakanyanya, hypotension, kutadza kwenhengo, uye kunyange mhedzisiro inouraya.
- Neurological Toxicity: Vamwe varwere vanogona kukudziridza mhedzisiro yetsinga, iyo inogona kusimuka mukuomarara kubva kune zvishoma zvakakomba zviratidzo senge kuvhiringidzika kunyoro uye kuvhiringidzika kune zvakakomba zvakanyanya senge pfari, delirium, uye encephalopathy. Mushure meCAR T-cell infusion, neurological toxicity inowanzoitika mukati mevhiki yekutanga.
- Cytopenias: CAR T-sero kurapwa kunogona kukonzera kuderera kwemasero eropa, akadai seanemia (yakaderera masero matsvuku eropa), neutropenia (yakaderera masero eropa eropa), uye thrombocytopenia (yakaderera platelet count). Utachiona, kubuda ropa, uye kupera simba ndedzimwe njodzi dzinogona kuwedzerwa neiyo cytopenias.
- Zvirwere: Iyo CAR T-cell kurapa kudzvanyirira kwemasero ane hutano ekudzivirira muviri kunowedzera njodzi yehutachiona, hutachiona, uye fungal utachiona. Kuti udzivise kutapukirwa, varwere vangangoda kutariswa zvakanyanya uye kupihwa mishonga yekudzivirira.
- Tumor Lysis Syndrome (TLS): Mushure meCAR T-cell therapy, zvinokwanisika mune mamwe mamiriro ezvinhu kuti huwandu hwakawanda hwemukati hubudiswe muropa nekuda kwekukurumidza kuuraya kwebundu maseru. Izvi zvinogona kukonzera kusagadzikana kwemetabolism, senge yakawandisa potassium, uric acid, uye phosphate mazinga, izvo zvinogona kukuvadza itsvo uye kukonzera mamwe matambudziko.
- Hypogammaglobulinemia: CAR T-sero kurapwa ine mukana wekudzikisa antibody synthesis, izvo zvinogona kukonzera hypogammaglobulinemia. Izvi zvinogona kuita kuti utachiona hunoramba huchidzokororwa huwedzere uye zvinodaidzira kuenderera mberi kwemishonga yekutsiva masoja ekudzivirira chirwere.
- Organ toxicity: CAR T-cell therapy ine mukana wekukuvadza nhengo dzakati wandei dzinosanganisira moyo, mapapu, chiropa, neitsvo. Izvi zvinogona kutungamira kune zvisina kujairika renal basa bvunzo, nyaya dzekufema, nyaya dzemoyo, uye kusakwana kwechiropa basa bvunzo.
- Hemophagocytic lymphohistiocytosis (HLH): Chirwere chisingawanzoitiki asi zvichida chinouraya chinouraya chinonzi hemophagocytic lymphohistiocytosis (HLH) chinogona kukura semugumisiro weCAR T-cell therapy. Inosanganisira kuwandisa kwemasero ekudzivirira muviri, izvo zvinokonzera kukanganisa kwakakomba kwenhengo uye kuzvimba.
- Hypotension uye Fluid Retention: Nekuda kweiyo cytokines iyo CAR T masero anoburitsa, vamwe varwere vanogona kukura yakaderera yeropa (hypotension) uye kuchengetwa kwemvura. Kugadzirisa zviratidzo izvi, matanho ekutsigira anosanganisira intravenous fluid uye mishonga inogona kudiwa.
- Secondary Malignancies: Mishumo yezvirwere zvechipiri zviri kubuda zvichitevera CAR T-cell therapy iripo, zvisinei nekushaikwa kwavo. Tsvagiridzo iri kuitwa parizvino pamusoro pekugona kwechipiri hutsinye uye njodzi dzenguva refu.
Zvakakosha kuyeuka kuti havasi murwere wese achava nemigumisiro iyi, uye kuti nhanho yemunhu wega wega inosiyana. Kuti uderedze uye uderedze zvinokonzeresa zvinokonzeresa izvi, timu yekurapa inoongorora varwere nguva isati yasvika, panguva, uye mushure meCAR T-cell therapy.
Time Frame
Tarisa pazasi nguva yakazara inodiwa kuti upedze iyo CAR T-Cell kurapwa maitiro. Kunyangwe nguva yakatarwa inotsamira zvakanyanya kureba kwerabhoritari kubva kuchipatara yakagadzira iyo CAR.
- Kuongorora & bvunzo: vhiki imwe
- Pre-kurapwa & T-Cell Kuunganidza: vhiki imwe
- T-Cell kugadzirira & kudzoka: mavhiki maviri-matatu
- 1st Kubudirira kuongororwa: mavhiki matatu
- 2nd Effectiveness analysis: mavhiki matatu.
Yese nguva yakatarwa: 10-12 Mavhiki
Tinogona Sei Kukubatsira Iwe Kuwana Yakanyanya Kurapa Kenza muIndia?
Kuwana kurapwa kwegomarara kwakanakisa muIndia kunogona kuve kwakaoma, kunyanya kana uchinetsekana nemutengo pamwe nemhando. Ndiko uko CancerFax inogona kukutungamira seshamwari yechokwadi!
Isu tinoziva kuti hutano hwako hwakakosha zvakanyanya uye kuti kukanganisa pamhando yekutarisira haisi sarudzo. Ndosaka takanyatsosarudza vanachiremba vane ruzivo rwakanyanya uye takabatana nezvipatara zvakati wandei pamitengo yakasiyana siyana kuitira kuti uwane zvakakunakira zvaunoda. Nenzira iyi, iwe unogona kugamuchira yakanakisa kutarisirwa pasina kutyora bhangi. Kwemakore gumi apfuura, nzira yedu yakatobatsira varwere vanobva kunyika dzinopfuura 10 huru, uye takazvipira kukuitirai zvimwe chetezvo. Vimba nesu kuti tigamuchire yakanakisa CAR T Cell kurapwa muIndia.
Zviri Nyore Maitiro Ekuwana CAR T Cell Therapy MuIndia
Tumira MaReports ako
Govera nhoroondo yako yekurapa, kusanganisira ichangoburwa mishumo yeropa, mhedzisiro yebiopsy, uye PET scans, nesu pa info@cancerfax.com. Iri danho rakakosha rinotibvumira kuongorora mamiriro ako uye kukutungamira iwe kune yakanyanya kufanirwa kurapwa.
Ongororo & Maonero
Chikwata chedu chenyanzvi chinonyatsoongorora mishumo yako kuti ipe ongororo yakakwana uye maonero enyanzvi. Izvi zvinotibatsira kuona nzira yakanaka yekuita uye kukurudzira zvipatara zvakanyanya uye nyanzvi dzeCAR T Cell therapy yako maererano nebhajeti yako.
Medical Visa Uye Kufamba
Isu tichakubatsira iwe mukuwana vhiza yekurapa uye ticharonga marongero ako ekufamba. Chinangwa chedu ndechekuita kuti rwendo rwako ruve nyore sezvinobvira kuitira kuti utarise kurapwa kwako uye kupora.
Kurapa Uye Kutevera
Kana wangosvika kuchipatara chawasarudza, timu yedu yakazvipira icharamba ichikubatsira panguva yese yekurapwa. Isu tinosimudzira kutaurirana pasina musono pakati pako nevashandi vezvokurapa, tichiva nechokwadi chekuti wawana kurapwa kwakanyanya. Kugarika kwako kunoramba kuchinyanya kukosha kwatiri.
Nyaya Dzesviro Dzidziso Kuv T-Cell Therapy In India
Chii chinonzi CAR T-cell therapy?
- CAR T-cell therapy imhando yeimmunotherapy inosanganisira kushandura masero eT emurwere kuti azive nekurwisa maseru egomarara. Kurapa kwemunhu uku kwakaratidza mhedzisiro inovimbisa mune mamwe marudzi egomarara.
Ko CAR T-cell therapy inowanikwa muIndia?
- Ehe, CAR T-cell therapy inowanikwa muIndia kune dzimwe nzvimbo dzegomarara. Nekudaro, kuwanikwa kwayo kunogona kusiyana, uye inogona kugumira kune mamwe marudzi egomarara.
Ndeapi magomarara anogona kurapwa neCAR T-cell therapy muIndia?
- Sezvekugadziridzwa kwangu kwekupedzisira, CAR T-cell therapy yainyanya kushandiswa kurapa mamwe marudzi egomarara reropa, akadai seleukemia nelymphoma. Iwo chaiwo cancers anokodzera kurapwa anogona kutsamira pane maprotocol anoteverwa neakasarudzika masangano ehutano.
Ndeipi mutengo weCAR T-cell therapy muIndia?
- Mutengo weCAR T-cell therapy unogona kunge wakakwira. Inogona kusanganisira mari yekuunganidza nekugadzirisa maT masero omurwere, maitirwo erabhoritari, uye mafambisirwo ekurapa. Mutengo unogona kusiyana zvichienderana nerudzi rwegomarara riri kurapwa uye nzvimbo yehutano.
Pane here mhedzisiro yeCAR T-cell therapy?
- Ehe, senge chero kurapwa, CAR T-cell therapy inogona kuve nemhedzisiro. Zvakajairwa mhedzisiro zvinosanganisira cytokine release syndrome (CRS) uye neurologic toxicities. Kuoma kwemigumisiro inogona kusiyana pakati pevanhu.
Inobudirira sei CAR T-cell kurapa mukurapa gomarara?
- CAR T-cell therapy yakaratidza kubudirira kunoshamisa mukurapa mamwe marudzi egomarara, kunyanya reropa. Zvisinei, kushanda kwayo kunogona kusiyana zvichienderana nerudzi uye danho rekenza, pamwe chete nemurwere mumwe nomumwe zvinhu.
Ko CAR T-cell therapy inovharwa neinishuwarenzi muIndia?
- Kuvharwa neinishuwarenzi kunogona kusiyana. Zvakakosha kutarisa nemupi weinishuwarenzi uye nzvimbo yehutano inopa kurapwa kuti unzwisise nzira dzekuvhara.
Ndingawana sei CAR T-cell therapy muIndia?
- Varwere vanofarira CAR T-cell therapy vanofanirwa kubvunza oncologists nevarapi vehutano vane hunyanzvi mukurapwa uku. Vanogona kutungamira varwere kuburikidza nemaitiro ekuongorora uye kuona kukodzera.