
Car - T Cell Lorem, CAR T Therapy, CAR T-, chimeric agente
A deep Dive in CAR T Cell Therapy: Quomodo operatur?
Scientiam post CAR T Cell curationem in India detege! Explorate quomodo haec curatio eversiva transmutat cellulas tuas immunes in pugnatores cancri. Read our blog now to learn more about this miraculosa therapy and how ..

Car - T Cell Lorem, CAR T-, Receptor Chimeric Agent, Korea, Seoul, South Korea
Societates in Corea gradum propius in explicando domo crevit, CAR T-Cell Lorem
May 2023 : Chimeric antigen receptor (CAR) T-cell Lorem sit amet eget in campo cancer individuied Lorem. Propriae T-cellulae aegri genere modificantur in processu fabricando ad exprimendum a..

Sinica, Car - T Cell Lorem, CAR T-, Currus Lorem, Sinis, fiscus itus, Oricell Therapeutica
Oricell$ 45M USD addito levat ut autocinetum T-Cell Lorem ad Civitates Foederatas extendat
Die 23 Martii 2023: Therapiae preclinicae et praematuro-cancri cellae Shanghai biotech Oricell amplificatae additae sunt $45 miliones in sumptu, societas die Martis nuntiata. Sequens ostendens in AS.,.

Australia, Car - T Cell Lorem, CAR T Therapy, Cartherics
Petrus MacCallum Cancer Centrum et Cartherics collaborabunt in cancer ovarii CAR-T cellam theraphim
Martii 2023: Peter MacCallum Cancer Centrum (Peter Mac) in Australia et Cartherics Pty Ltd inierunt cooperationem programmatis fovendi (CDPA) evolvere CTH-002 ad curationem cancri ovarii. The cli.,.

Car - T Cell Lorem, CAR T-, Aemilia Littlejohn, Inmunología, lupus renaissance
Novum CAR T-Cell illic pharmacum in lupus renaissance
Feb 2024: Plures medicamenta nova et therapiae promittens, ut antigenum receptaculum chimaricum T-cell therapiae, in "renaissance" lupus induxerat, secundum oratorem in symposio Basic et Fusce immunologia pro Busy ..
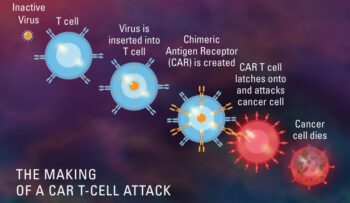
anti-BCMA, Car - T Cell Lorem, CT103A, FDA, IASO Biotherapeutics, Innovent Biologics, pupillus medicamento designatio
CT103A, CAR T-cell Therapy, medicamentum orphanum a FDA designatum est
Feb 2023: The FDA has granted orphan drug status to CT103A, an experimental CAR T-cell therapy being developed by IASO Biotherapeutics and Innovent Biologics to treat relapsed or refractory multiple myeloma. Orphan drug design..
Car - T Cell Lorem, Sinis, IASO Biotherapeutics, multa myeloma
Curatio autocinetica T-cellula ab IASO biotherapeutica novam FDA approbationem accipit
Feb 2023: IASO Inquisitionis biotherapeuticae CAR T-cell therapiae pro relapso vel refractorio multiplex myeloma (RRMM), CT103A, ieiunium semitam accepit et medicinam regenerativam a curatione US Cibus et designationibus provexit.
Car - T Cell Lorem, Seoul, South Korea
AMC aperit autocinetum T-Cell Lorem centrum in Seoul
Ian 2023: Asan Medical Centre (AMC) primum CAR-T cellam curationis facilitas in patria aperuit post regimen sanitatis assecurationis approbatae pro Kymriah's CAR-T cellam curationis.AMC die Martis nuntiatum est suam canc.
Sanguinem test, Car - T Cell Lorem, JAMA Oncology, lux catenae neurofilamentum, Neurotoxic Complicationes, Washington University
CAR T-CELL Lorem inpedimenta per simplicem sanguinem test
Sept. 2022: Curatio variorum tumorum per cella substructio immunotherapy, saepe nota sicut therapia CAR-T cellula mutata est. Ad oppugnandum et pugnandum formas specificas leukemia et lymphoma, curatio genetica adhibet.
Car - T Cell Lorem, CARsgen Therapeutica Co, Daiichi Sankyo Company, DLBCL, Emergen Research, stricto, laboratorium Novartis AG
CAR T-Cell Lorem forum in phaenomenis crescet in proximis 8 annis
Iulii 2022: Secundum recentissimam investigationem ab Emergen Research deductam, mercatum globalis pro CAR T-cellulis illic molis USD 1.29 miliardis 2021 attigit et vectigal CAGR 24.9 centesimis mandare expectatur.